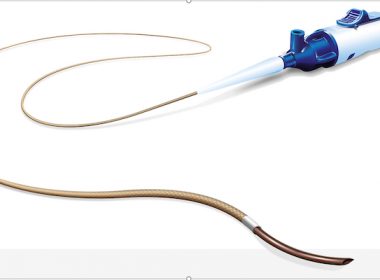
ST. PAUL, Minn.–(BUSINESS WIRE)–Cardio Flow, Inc., a medical device company and developer of minimally invasive peripheral vascular devices to treat peripheral artery disease (PAD), today announced it recently received U.S. Food and Drug Administration (FDA) clearance for the company’s FreedomFlow® Peripheral Guidewire. The FreedomFlow guidewire is stainless steel core-to-tip design […]
Peripheral/Endo
Silk Road Medical Announces Expanded Medicare Coverage for TCAR in Standard Surgical Risk Patients
SUNNYVALE, Calif., June 02, 2022 (GLOBE NEWSWIRE) — Silk Road Medical, Inc. (Nasdaq: SILK), a company focused on reducing the risk of stroke and its devastating impact, today announced that the Centers for Medicare and Medicaid Services (CMS), through collaboration with the Society of Vascular Surgery’s Patient Safety Organization (SVS PSO) […]
Cagent Vascular Announces Commercial Launch of the Serranator Above-the-Knee (ATK) Product
WAYNE, Pa.–(BUSINESS WIRE)–Cagent Vascular Inc., a developer of next generation angioplasty balloons using serration technology, announced the expansion of its product offering to include larger balloons to treat superficial femoral and popliteal arteries in the Above-the-Knee (ATK) segment. Cagent Vascular launched the Serranator® Below-the-Knee (BTK) product in January 2021. Since then, […]
Medtronic low-profile drug-coated balloon platform receives U.S. FDA approval to treat peripheral arterial disease
Built on the market-leading IN.PACT™ Admiral™ DCB technology, the IN.PACT™ 018 DCB is engineered to cross tight lesions and designed for better deliverability§ Medtronic today announced approval from the U.S. Food and Drug Administration (FDA) for the IN.PACT™ 018 Paclitaxel-Coated Percutaneous Transluminal Angioplasty (PTA) Balloon Catheter, a drug coated balloon […]
MedAlliance’s SELUTION SLR drug eluting balloon (DEB) receives FDA investigational device exemption (IDE) approval – making it the first limus DEB to be available to US patients
GENEVA, May 30, 2022 /PRNewswire/ — The SELUTION SLR (Sustained Limus Release) is a novel sirolimus-eluting balloon that provides a controlled sustained drug release, similar to a drug-eluting stent (DES). SELUTION SLR was also the first DEB (Drug Eluting Balloon) granted “breakthrough device designation” by the FDA on March 4, 2019 and further in September […]
Vascular Access Experts to Tackle Ultrasound Disinfection Controversy at INS 2022
Presentation will highlight development of recent recommendation for low-level disinfection of transducers used in percutaneous procedures HARTWELL, Ga., May 26, 2022 /PRNewswire/ — A presentation at the upcoming annual meeting of the Infusion Nurses Society (INS) will discuss the recent development of a policy statement favoring low-level disinfection (LLD) as a safe […]
VentureMed Completes Enrollment of FLEX Vessel PrepTM System Randomized Control Trial in Arteriovenous Fistulas
MINNEAPOLIS, May 24, 2022 /PRNewswire/ — VentureMed Group, Inc., a privately held medical device innovator in access management for arteriovenous (AV) fistulas and grafts and vessel preparation for interventional treatment of peripheral arterial disease (PAD) announced today that it has completed enrollment of a randomized clinical trial (RCT) titled “FLEX Vessel Prep Prior To […]
Upstream Peripheral Technologies Partners with Red One Medical to Facilitate Access of the GoBack Catheter to the VA Health System
TAMPA, Fla.–(BUSINESS WIRE)–Upstream Peripheral Technologies announced today that it has partnered with Red One Medical for the introduction of the GoBack® Crossing and Reentry Catheter to the VA Health System. The partnership will allow physicians in the VA Health System to have access to this new technology in treating patients […]
Low stroke risk in patients with very narrowed neck arteries
Kaiser Permanente study suggests that for patients with severe asymptomatic carotid stenosis who do not have surgery medical management alone may reduce stroke risk OAKLAND, Calif. , May 24, 2022 /PRNewswire/ — The risk of having a future stroke caused by a severe blockage in an artery in the neck that is not […]
United Therapeutics Announces FDA Approval of Tyvaso DPI™
First approval of a dry powder inhaler for treatment of PAH and PH-ILD DPI device represents a convenient option for administration of treprostinil therapy Commercial launch activities underway; patient availability expected in June 2022 SILVER SPRING, Md. & RESEARCH TRIANGLE PARK, N.C.–(BUSINESS WIRE)–United Therapeutics Corporation (Nasdaq: UTHR), a public benefit […]