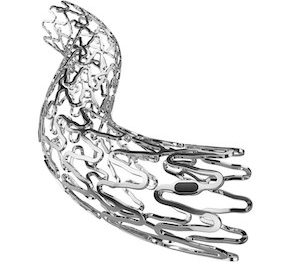
MONTREAL–(BUSINESS WIRE)–Soundbite Medical Solutions Inc. (SBMS), a medical device company dedicated to developing solutions for the interventional treatment of calcific peripheral and coronary arterial diseases, today announced the use of its novel Active Wire 0.014” platform at a prominent site in Germany with the successful treatment of patients suffering from heavily calcified […]
Peripheral/Endo
RapidAI Launches Rapid Workflow for Pulmonary Embolism (PE)
Developed in partnership with Penumbra, Inc., Rapid Workflow for PE supports faster team activation to accelerate PE care and drive better patient outcomes SAN MATEO, Calif., June 24, 2021 /PRNewswire/ — RapidAI, leading the next evolution of clinical decision making and patient workflow, today announced the availability of Rapid Workflow for PE, a mobile […]
Peijia Medical’s Jasper® SS Detachable Coil Receives NMPA Approval for Commercialization
SUZHOU, China, June 24, 2021 /PRNewswire/ — On June 15, 2021, Jasper® SS—a new detachable coil independently developed by Achieva Medical, subsidiary of Peijia Medical (9996.HK)—was approved by the National Medical Products Administration (“NMPA”) for commercialization (registration number GXZZ 20113130440). In the field of neurointervention, embolization coils are designed to be as thin as […]
JanOne Readies Clinical Supply of Lead Product Candidate JAN101 for Distribution to Phase 2b Trial Sites
Company Fully Prepared to Commence Phase 2b Trials of JAN101 for Peripheral Artery Disease (PAD) as Soon as New Protocol is Approved by the FDA LAS VEGAS, June 23, 2021 /PRNewswire/ — JanOne Inc. (Nasdaq: JAN), a company focused on developing treatments for conditions that cause severe pain and drugs with non-addictive, pain-relieving properties, […]
Penumbra Announces FDA Clearance and Commercial Availability of RED 62 Reperfusion Catheter
Now available in the U.S., RED™ 62 is engineered with optimized trackability to help navigate the complex distal vessel anatomy in the brain and deliver powerful aspiration for the removal of blood clots First catheter of new RED series expands the company’s extensive portfolio of innovative stroke products ALAMEDA, Calif.–(BUSINESS WIRE)–Penumbra, […]
JanOne Selects Regulatory Partner for Phase 2b Trial as Investigational Plan is Prepared for FDA Filing
Global Contract Research Organization (CRO) Avania to Manage All Regulatory Affairs in Connection with JanOne’s Phase 2b Trial Preparedness and Execution LAS VEGAS, June 22, 2021 /PRNewswire/ — JanOne Inc. (Nasdaq: JAN), a company focused on developing treatments for conditions that cause severe pain and drugs with non-addictive, pain-relieving properties, today announced its selection […]
QualiMed Innovative Medizinprodukte GmbH, a Q3 Medical Devices Ltd Company, Receives European CE Mark Approval for UNITY-B™ Percutaneous Balloon Expandable Biodegradable Biliary Stent
Novel Biodegradable Stent Technology Approved for Sale in Europe WINSEN, Germany, June 21, 2021 /PRNewswire/ — QualiMed Innovative Medizinprodukte GmbH, a wholly owned subsidiary of Dublin, Ireland based Q3 Medical Devices Limited (Q3), announced that it has received CE mark approval for a dedicated percutaneous biodegradable metal alloy based stent implant for biliary applications, the UNITY-B Percutaneous Balloon […]
JanOne Advances Toward Initiation of Phase 2b Peripheral Artery Disease (PAD) Trial for Lead Product Candidate JAN101
Successfully Completes Initial Batch Production of JAN101, Demonstrating CGMP-scale Production Capabilities LAS VEGAS, June 8, 2021 /PRNewswire/ — JanOne Inc. (Nasdaq: JAN), a company focused on developing treatments for conditions that cause severe pain and drugs with non-addictive, pain-relieving properties, today announced the successful completion of Current Good Manufacturing Practices (CGMP) production of […]
Arena Pharmaceuticals Announces First Participant Randomized in Phase 2 Trial Evaluating Temanogrel in Coronary Microvascular Obstruction (cMVO)
– Evaluating the safety, tolerability, and efficacy of intravenous (IV) temanogrel for cMVO in adult participants undergoing percutaneous coronary intervention (PCI) – Currently no FDA approved therapies indicated for the treatment of cMVO – Temanogrel was granted FDA Fast Track Designation for the treatment of cMVO PARK CITY, Utah–(BUSINESS WIRE)–Arena […]
Alnylam Announces New Data from ATTR Amyloidosis Programs at the Peripheral Nerve Society’s 2021 Annual Meeting
− Phase 3b Open-Label Study Showed Treatment with Patisiran Achieved Rapid and Sustained Reduction in Serum TTR Levels in hATTR Amyloidosis Patients with Polyneuropathy Progression Following Orthotopic Liver Transplant – − Additional Results from Pre-specified Patient Subgroups Analysis Included with Encore Presentation of HELIOS-A Phase 3 Study of Investigational Vutrisiran […]