– Results were published in the Journal of Vascular Surgery –
Peripheral/Endo
Late Breaking Clinical Trial from AVS IVL Pivotal Study to be Presented at VIVA
POWER PAD II IDE trial evaluates safety and efficacy of the Pulse IVL™ system in patients with severely calcified peripheral arterial disease BOSTON–(BUSINESS WIRE)–Amplitude Vascular Systems (AVS), a medical device company focused on safely and effectively treating severely calcified arterial disease, announced today that it will present 30-day results of its […]
Vascular Innovation on Display: The VIVA Foundation Set to Unveil Latest Trial Data at The VEINS and VIVA 2025
PALM SPRINGS, Calif., Sept. 10, 2025 /PRNewswire/ — The VIVA Foundation will once again showcase the most influential new data in vascular medicine on a global stage. From Saturday, November 1, through Tuesday, November 4, results from 23 clinical trials will be presented during The…
Shape Memory Medical Completes Enrollment in the EMBO-Post Market Surveillance Registry
SAN JOSE, Calif.–(BUSINESS WIRE)–Shape Memory Medical Inc., the innovator of shape memory polymer for endovascular applications, announced the completion of patient enrollment in the EMBO Post Market Surveillance (EMBO-PMS) Registry, the Company’s prospective, multicenter registry of the IMPEDE and IMPEDE-FX Embolization Plugs, and IMPEDE-FX RapidFill Device when used for peripheral […]
Microbot Medical® Receives FDA 510(k) Clearance for Its LIBERTY® Endovascular Robotic System
Microbot Medical® Receives FDA 510(k) Clearance for Its LIBERTY® Endovascular Robotic System.
Silent Vascular Threat: Nearly 70% of Americans Unaware of PAD – The Leading Cause of Preventable Amputations
During PAD Awareness Month, the Society for Vascular Surgery Urges Americans to Learn How to Safeguard Their Vascular Health ROSEMONT, Ill., Sept. 8, 2025 /PRNewswire/ — Despite vascular disease dominating headlines in recent months—from prominent public figures with visible symptoms and…
ASAHI INTECC Launches Veloute™ and Tellus™ Embolization Microcatheters in the U.S.
Next-generation deliverability and control for peripheral embolization — available September 8, 2025 IRVINE, Calif., Sept. 8, 2025 /PRNewswire/ — ASAHI INTECC today announced the U.S. commercial launch of Veloute™ and Tellus™. These medical devices are indicated for angiography and…
FLEX Vessel Prep™ System Spotlighted for Clinical Outcomes, Staff Efficiency Gains, and Real-World Physician Experience in the Journal of Vascular Access, Vascular News, and Endovascular Today
MINNEAPOLIS, Sept. 4, 2025 /PRNewswire/ — VentureMed Group, Inc., a privately held leader in medical device innovations for arteriovenous (AV) access and peripheral arterial disease (PAD), was recently featured in a published study on the outcomes of FLEX VP + DCB in the Journal of…
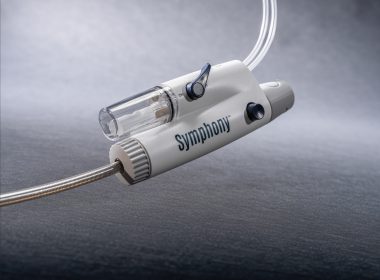
Imperative Care Announces FDA Clearance of Symphony Thrombectomy System, the First Large-Bore Continuous Vacuum System for Treatment of Pulmonary Embolism
CAMPBELL, Calif.–(BUSINESS WIRE)–Imperative Care, Inc. today announced U.S. Food and Drug Administration (FDA) 510(k) clearance of its Symphony® Thrombectomy System to treat pulmonary embolism (PE), a life-threatening condition caused by blood clots blocking an artery in the lungs. This clearance expands the use of Symphony – previously for the treatment of […]
Phraxis Announces First-Ever Commercial Case of EndoForce™ Anastomotic Connector at Spartanburg Regional Medical Center
MINNEAPOLIS, Aug. 29, 2025 /PRNewswire/ — Phraxis, Inc., a leader in vascular access innovation, today announced the successful completion of the first commercial case using the EndoForce™ Anastomotic Connector, marking a historic milestone in the treatment of dialysis patients worldwide….