First-of-its-kind medical device aims to improve vascular access for hemodialysis patients PRAIRIE VILLAGE, Kan., Jan. 27, 2021 /PRNewswire/ — Artio Medical, Inc., a medical device company developing innovative products for the peripheral vascular, neurovascular, and structural heart markets, announced today that it has successfully completed the first human use of its Amplifi™ Vein Dilation […]
Peripheral/Endo
FLEX Vessel Prep™ System Data Presented During Leipzig Interventional Course (LINC) 2021
Trio of studies demonstrate that FLEX Vessel Prep System safely and effectively modifies obstructive plaque to facilitate delivery of definitive therapies MINNEAPOLIS, Jan. 27, 2021 /PRNewswire/ — VentureMed Group, Inc. (VentureMed), a privately-held medical device innovator, announced today that data from three studies evaluating the use of its FLEX Vessel Prep (VP) System were […]
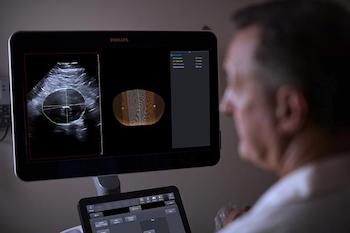
Philips integrates 3D ultrasound with innovative software for breakthrough in surveillance of abdominal aortic aneurysms
Philips Abdominal Aortic Aneurysm (AAA) Model helps increase diagnostic confidence and improved patient experience compared to current standard of care Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, has introduced the Philips Abdominal Aortic Aneurysm (AAA) Model, providing physicians a more patient-friendly solution compared to […]
SoundBite Medical Solutions Announces First Use of its Novel 0.014” Active Wire to Successfully Treat Calcified Below-The-Knee CTOs
MONTREAL–(BUSINESS WIRE)–SoundBite Medical Solutions Inc. (SBMS) announced today the first use of its novel Active Wire 0.014” platform in the successful treatment of patients suffering from critical limb ischemia (CLI) with heavily calcified below-the-knee (BTK) chronic total occlusions (CTO). The procedures were performed by Professor Marianne Brodmann, Head of the […]
12-Month Data from Surmodics’ TRANSCEND Trial Presented at LINC 2021 Event
SurVeil™ Drug Coated Balloon (DCB) demonstrates non-inferior safety and efficacy, while using a substantially lower drug dose, vs. the IN.PACT® Admiral® DCB for treatment of femoropopliteal lesions. EDEN PRAIRIE, Minn.–(BUSINESS WIRE)–Surmodics, Inc. (NASDAQ:SRDX), a leading provider of medical device and in vitro diagnostic technologies to the health care industry, today announced that […]
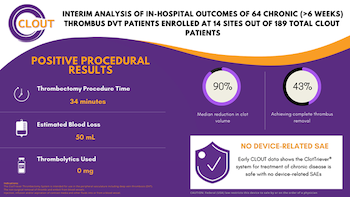
Inari Medical Announces Presentation of Positive Chronic Clot Subanalysis Results from Real World CLOUT Registry at LINC 2021
IRVINE, Calif., Jan. 25, 2021 (GLOBE NEWSWIRE) — Inari Medical, Inc. (NASDAQ: NARI) (“Inari”) a commercial-stage medical device company focused on developing products to treat and transform the lives of patients suffering from venous diseases, today announced strongly positive interim results of the first 64 chronic deep vein thrombosis (“DVT”) […]
PEDRA™ Technology Receives FDA Breakthrough Device Designation for its PEDRA™ Xauron™ Real-Time Tissue Perfusion System
Novel perfusion monitor achieves FDA Breakthrough Device Designation for real-time, periprocedural monitoring of tissue perfusion in patients with critical limb threatening ischemia SINGAPORE, Jan 25, 2021 /PRNewswire/ — PEDRA™ Technology, a privately-held company, announced today that the U.S Food and Drug Administration (FDA) has granted the company a Breakthrough Device Designation for the […]
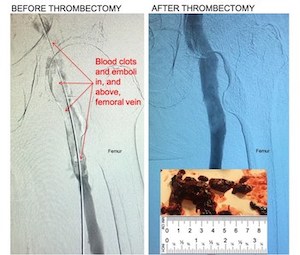
Kishor Vora, M.D. Owensboro Heart & Vascular, Removes Large Blood Clots in 1st Global Use of New Device
11F mechanical thrombectomy system removes large blood clots in deep vein thrombosis (DVT) HALLANDALE BEACH, Flo., Jan. 22, 2021 /PRNewswire/ — Control Medical Technology announced the FDA cleared Control 11F Mechanical Thrombectomy system was used to remove large blood clots from patients with deep vein thrombosis (DVT). “Control removed large blood […]
JACC Study: PEN Indigo Aspiration System Meets Safety/Efficacy Endpoints
A study published online first in the JACC: Cardiovascular Interventions found that Penumbra, Inc.’s Indigo Aspiration System met its predefined safety and efficacy endpoints for the treatment of pulmonary embolism (PE) in the EXTRACT-PE study. Specific results include a significant mean reduction in right ventricular (RV)/left ventricular (LV) ratio of […]
Teleflex continues to deliver COMPLETE confidence with new Arrow® ErgoPack® Complete MAC, PSI Systems
Teleflex expands value of ErgoPack® Complete enhancements to critical vascular access products WAYNE, Pa., Jan. 19, 2021 (GLOBE NEWSWIRE) — Teleflex Incorporated (NYSE: TFX) continues to deliver COMPLETE confidence with the release of the Arrow® ErgoPack® Complete Multi-Lumen Access Catheter (MAC) System and the Arrow® ErgoPack® Complete Percutaneous Sheath Introducer (PSI) System. The release […]