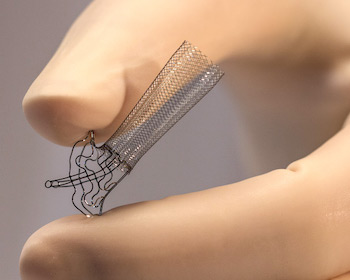
HINGHAM, Mass., Jan. 08, 2020 (GLOBE NEWSWIRE) — Microbot Medical Inc. (Nasdaq: MBOT) has received an official communication from the European Patent Office (EPO) regarding its intention to grant European Patent Application No. 11795301, covering the Company’s Self-Cleaning Shunt (SCS™). Globally, the Company now has 36 patents issued/allowed and 16 […]
Peripheral/Endo
Vascular Graft Solutions Acquires the Intellectual Property Developed by Kipsbay Medical Inc.
TEL AVIV, Israel, Jan. 6, 2020 /PRNewswire/ — Vascular Graft Solutions (VGS) announces on the acquisition of several patent families from NEOGRAFT Technologies Inc. which include patents that were previously developed and owned by Kipsbay Medical Inc, a pioneer in the field of venous external stenting and vascular engineering. The patents portfolio, which includes […]
CMS Approves Coverage for PQ Bypass TORUS 2 IDE Trial
Latest accomplishment reached by Silicon Valley med-tech company currently enrolling two IDE studies MILPITAS, Calif.–(BUSINESS WIRE)–PQ Bypass Inc, a medical device company bringing new advancements to the treatment of peripheral artery disease (PAD), announced today that it has received approval for coverage from the Centers for Medicare and Medicaid Services […]
Results Demonstrating Favorable Outcomes with TCAR vs. TF-CAS in Patients with Carotid Artery Stenosis Published in the Journal of the American Medical Association (JAMA)
SUNNYVALE, Calif., Dec. 17, 2019 (GLOBE NEWSWIRE) — Silk Road Medical, Inc. (Nasdaq: SILK), a company focused on reducing the risk of stroke and its devastating impact, today announced that positive results from the ongoing TransCarotid Artery Revascularization (TCAR) Surveillance Project comparing TCAR and transfemoral carotid artery stenting (TF-CAS) have been published in The Journal […]
MemorialCare Orange Coast Medical Center Offers TCAR Procedure to Treat Carotid Artery Disease
FOUNTAIN VALLEY, CA – MemorialCare Orange Coast Medical Center has announced that it is now offering a new procedure called TransCarotid Artery Revascularization (TCAR) to treat carotid artery disease and prevent future strokes. TCAR is a clinically proven, minimally invasive and safe approach for high surgical risk patients who need […]
Cardiovascular Systems, Inc. Introduces Peripheral Orbital Atherectomy System With GlideAssist® in Europe
Dr. Michael Lichtenberg Treats First Patient in Arnsberg, Germany ST. PAUL, Minn.–(BUSINESS WIRE)–Cardiovascular Systems, Inc. (CSI®) (NASDAQ: CSII), a medical device company developing and commercializing innovative interventional treatment systems for patients with peripheral and coronary artery disease, announced today that the first patient in Europe has been treated with its […]
Enrollment Complete for VALUE, the Prospective EU VasQ™ External Support Post-Market Study
TEL AVIV, Israel, Dec. 11, 2019 /PRNewswire/ — Laminate Medical Technologies (Laminate) has announced the completion enrollment of the VALUE study for the VasQ™ External Support. The post-market study enrolled 80 patients (50 upper arm and 30 forearm fistulas) for sites across Germany, France, Spain and the UK and will be followed for one year. The study is the […]
CORRECTING and REPLACING First Patient Enrolled in PROMISE II U.S. Pivotal Study of LimFlow System to Treat Chronic Limb-threatening Ischemia
Clinical Study to Evaluate Device’s Ability to Prevent Amputation and Promote Wound Healing in Patients with No Other Options CORRECTION…by LimFlow SA PARIS–(BUSINESS WIRE)–First paragraph, last sentence should read: The successful first case was performed by Mark Archie, MD, principal investigator for the PROMISE II trial at Harbor-UCLA Medical Center, […]
Canon Medical’s Alphenix Platform at the Forefront of Helping Hospitals Prepare for the Future of Interventional Medicine
Canon Medical to Showcase High-Definition Image Resolution Technology at RSNA 2019 CHICAGO–(BUSINESS WIRE)–Since its launch in 2018, Canon Medical’s Alphenix™ platform has helped clinicians think beyond the traditional boundaries of interventional procedures, with its streamlined workflow, dose optimization and improved image resolutions. For example, the specialized team of neuroendovascular surgeons […]
Shape Memory Medical Receives PMDA Approval for the IMPEDE® Embolization Plug Family
SANTA CLARA, Calif.–(BUSINESS WIRE)–Shape Memory Medical Inc. announced today that its IMPEDE® Embolization Plugs have been approved in Japan by the Pharmaceuticals and Medical Devices Agency (PMDA). Cosmotec, which is a Group Company of M3 Inc https://corporate.m3.com/en/ listed on the Tokyo Stock Exchange (TYO:2413), championed the approval process in Japan and is […]