Caesarea, Israel – December 14, 2023 – Arineta Cardio Imaging, a leader in advancing cardiovascular imaging solutions through cutting-edge technology, installed its first SpotLight scanner in the U.S. at Connected Cardiovascular Care Associates (C3) in Dallas. Arineta ushers in this milestone along with the appointment of its new CEO, Douglas […]
Radiology
SimonMed Imaging Unveils New Data on Leveraging Artificial Intelligence for Decreased Turnaround Time and Increased Accuracy on MSK X-Ray Readings
SCOTTSDALE, Ariz., Dec. 13, 2023 – SimonMed Imaging (“SimonMed”), one of the largest outpatient medical imaging providers and radiology practices in the United States, recently unveiled new study data at the 2023 Radiological Society of North America’s Radiology Conference and Annual meeting. The original study evaluated the impact of Artificial […]
4DMedical and Imbio Merge to Create Comprehensive Cardiothoracic Image Analysis Portfolio
Acquisition brings complementary line of advanced analytical imaging technologyLOS ANGELES, Dec. 11, 2023 /PRNewswire/ — 4DMedical announces it has entered a binding agreement to acquire Imbio (a portfolio company of Minneapolis-based Invenshure). As the creators of groundbreaking lung imaging technologies, 4DMedical aims to revolutionize respiratory imaging and ventilation— for improved lung disease detection and management. Imbio technology complements those products perfectly, delivering quantitative and personalized imaging analysis for patients suffering from both acute and chronic diseases.
Core to both companies is a desire to improve the way lung disease is detected and subsequently managed. The acquisition aligns with 4DMedical’s growth strategy, providing physicians with a comprehensive suite of products combining structure and function in assessing lung disease, effectively “owning the lung.”
4DMedical XV Technology® current and pipeline software products provide regional quantification of lung function using existing imaging equipment, or the dedicated XV Scanner. Imbio’s fully automated technology transforms chest CT studies into rich visual maps of a patient’s lungs. Accompanying reports provide detailed data on the type and extent of abnormalities found in the images. Imbio algorithms support multiple clinical initiatives such as lung cancer screening, smoking cessation, surgical planning, and pulmonary embolism management programs—and can be used in clinical trials and academic research for numerous diseases.
Combining 4DMedical and Imbio existing technologies into a unified offering and development platform will drive respiratory health improvements around the globe. Imbio’s current portfolio provides validated and market-leading insights to highly specialized healthcare providers. By combining this toolset with 4DMedical’s novel technology and automated point-of-care radiology workflows, 4DMedical will revolutionize lung disease diagnosis by making it simultaneously more comprehensive, objective, personalized, and, most importantly, more widely available.The combined technology offering holds the potential to turn any standard Chest CT into a much broader Cardiothoracic Analysis—immediately providing functional, structural, and risk-based analysis for both lung and heart disease.4DMedical’s commercialization of XV Technology® in the US will benefit from the opportunity to be delivered into established Imbio contracts. This opens up the exciting opportunities of providing patient screening programs for chronic obstructive pulmonary disease (COPD), interstitial lung disease (ILD), lung cancer and heart disease in the Australian and US markets.4DMedical Founder and CEO Dr. Andreas Fouras said, “We are extremely pleased with the acquisition of Imbio; it is a highly complementary strategic fit for 4DMedical, expanding our addressable market. Imbio’s suite of products deploy AI solutions to add quantification and visualization to lung structure from CT scans, bringing new indications to 4DMedical. The acquisition also provides additional inroads to engage with the United States Department of Veterans Affairs, boosting 4DMedical’s mission to improve healthcare for Veterans.””Imbio is very excited to join forces with 4DMedical to offer expanded capabilities and customer support. Together we can help clinicians utilize the vast quantitative information obtained from patients’ diagnostic chest images for lung pathologies to ultimately drive actionable decision-making for better patient care,” said Dave Hannes, CEO at Imbio.About ImbioImbio is a leader in artificial intelligence (AI) medical imaging solutions for chronic lung and cardiothoracic diseases. Imbio’s regulatory cleared solutions transform the way patients are discovered, diagnosed, and treated, enabling physician productivity and more personalized care for patients. For more information, please visit www.imbio.comAbout 4DMedical4DMedical Limited (ASX:4DX) is a global medical technology company that has created a step change in the capacity to accurately and quickly understand the lung function of patients with respiratory diseases.Through its flagship patented XV Technology®, 4DMedical enables physicians to understand regional airflow in the lungs and identify respiratory deficiencies earlier and with greater sensitivity as they breathe. This technology powers 4DMedical’s FDA-cleared XV Lung Ventilation Analysis Software (XV LVAS®)—the first modality to dynamically quantify ventilation throughout the lungs, and its Computed Tomography-enabled counterpart software, CT LVAS™.XV LVAS and CT LVAS reports are prepared using 4DMedical’s Software as a Service delivery model using existing hospital imaging equipment or the Company’s revolutionary XV Scanner.To learn more, please visit www.4dmedical.com.Contact:Colin Reed[email protected]SOURCE 4DMedical
ABK Biomedical announces that its Eye90 microspheres® device has been granted Breakthrough Device Designation by the U.S. Food and Drug Administration (FDA)
HALIFAX, NS, Dec. 5, 2023 /PRNewswire/ – ABK Biomedical, Inc., an innovative, medical device company dedicated to the research, development, and commercialization of advanced imageable embolic therapies, announces its Eye90 microspheres device has been granted Breakthrough Device Designation by the U.S. Food and Drug Administration (FDA). This designation is for the proposed Eye90 microspheres indication for the treatment of patients living with unresectable Hepatocellular Carcinoma (HCC). Breakthrough designation is granted to devices that may provide more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions for which no approved alternative exists.
Continue Reading
“We are pleased with the FDA’s decision to grant Breakthrough Device Designation for Eye90 microspheres Y90 radioembolization device. This confirms our belief that Eye90 represents an important evolution of radioembolization technology with the potential to significantly improve patient outcomes. Our discussions with the FDA have been productive, and this designation will allow us to streamline interactions with FDA and bring this product to market in an efficient manner. We look forward to executing our Route90 trial and our continued collaboration with the FDA,” said Mike Mangano, President, and CEO of ABK Biomedical.
ABK Biomedical’s Eye90 microspheres® device has been granted Breakthrough Device Designation by the FDA
Post this
ABK Biomedical recently initiated enrollment in Route90, an FDA IDE approved pivotal, prospective, multicenter trial to establish the safety and efficacy of Eye90 microspheres in patients with unresectable HCC.
Dr. Aravind Arepally, Chief Medical Officer of ABK Biomedical, stated, “Eye90 microspheres radioembolization marks a significant breakthrough in the treatment of HCC. This medical device allows us to leverage multi-modality imaging, facilitate controlled visual administration and offers personalized dosimetry. This gives us the opportunity to further advance the field of Y90 radioembolization. We’re excited that FDA also recognizes the potential of ABK’s technology to improve patient outcomes.”
About FDA Breakthrough Devices Program
The FDA Breakthrough Devices Program is a voluntary program for certain medical devices and device-led combination products that provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. It is available for devices and device-led combination products which are subject to review under a premarket approval application (PMA), premarket notification (510(k)), or De Novo classification request (De Novo request). This program is intended to help patients have more timely access to these medical devices by expediting their development, assessment, and review, while preserving the statutory standards for PMA approval, 510(k) clearance, and De Novo marketing authorization, consistent with the FDA’s mission to protect and promote public health.
About ABK Biomedical, Inc.
ABK Biomedical is a company focused on researching, developing and commercializing medical device therapies to improve treatment outcomes and the lives of patients with benign and malignant hypervascular tumors. ABK Biomedical holds intellectual property in the areas of inorganic polymer microspheres and unique administration systems. The company possesses advanced intellectual capital and its own R&D and manufacturing facilities for developing and commercializing unique embolotherapy products. Eye90 microspheres® is considered an investigational product and is not approved for use in any regulatory jurisdiction outside of approved clinical trials. SOURCE ABK Biomedical Inc.
MDView Expands Radiology Second Opinion Services to Additional States and Announces New Medical Director as Part of Partnership with Modern Teleradiology
The second opinion platform expands radiology second opinion services beyond Florida to accept patients in 12 US states and Canada while appointing Dr. Sunil Kini as Medical Director.COOPER CITY, Fla., Dec. 5, 2023 /PRNewswire/ — MDView, an end-to-end medical second opinion platform, announces a new partnership with Modern Teleradiology, LLC and their board certified and subspeciality radiologists to provide second opinion reports and video consultations to users of the MDView platform. In addition to the new partnership, Modern Teleradiology founder and CEO, Sunil Kini, MD, was appointed as Medical Director of MDView to aid in the platform’s expansion of features aimed at streamlining second opinion services.
Continue Reading
Radiology Second Opinions – Connect with expert radiologists to get a second opinion report on medical imaging exams.
Dr. Kini and his team will play a crucial role in helping MDView reach more patients by affiliating with organizations seeking to improve patient care. This includes offering second opinions to reduce unnecessary procedures, expedite accurate diagnoses, and lower overall costs.
Modern Teleradiology joins the growing list of radiologists providing services via the MDView platform. Initially launched in June 2023, MDView’s expansion now offers radiology second opinions in Florida, New York, Texas, California, Arizona, Pennsylvania, Alabama, Illinois, Georgia, Montana, Nevada, Tennessee, and Canada, including subspeciality breast imaging second opinions in Pennsylvania and Florida.
Tracy Amato, CEO of MDView, emphasizes the mission to provide patients with peace of mind regarding their diagnosis. She states, “A patient’s radiology report is the foundation of their diagnosis and treatment plan. By having a radiologist provide an expert opinion report, patients and their doctors can be sure they are making the right decisions for a better outcome. We are delighted to have Dr. Kini join us in executing this mission and fueling the MDView platform with his team of expert radiologists to support our expansion.””We are thrilled to be collaborating with MDView as both organizations share the same ‘patient first’ vision,” said Dr. Kini. “These days, patients are more empowered regarding their own health. To that end, MDView’s second opinion platform aims to give patients more transparency when it comes to making decisions regarding their own well-being. We are excited to join MDView in our mission to make healthcare more patient-centric.”Philip Pullum, COO of Modern Teleradiology, adds, “We are very excited about our collaboration with MDView. We wholeheartedly support patient advocacy and embrace the ability to assist patients in the direction of their own care.”Beyond radiology second opinion services, the MDView platform offers plans to employers of any size as supplemental benefits to their current health plans. This allows employees virtual access to verify findings in their radiology imaging exams when dealing with new or uncertain medical conditions or before proceeding with invasive surgery or treatments. Additionally, MDView customizes its second opinion platform for medical institutions and practices looking to implement a turn-key second opinion program.Patients interested in getting a radiology second opinion can register for a free MDView account at https://app.mdview.com. For more information about MDView’s offerings, visit https://mdview.com.About MDViewMDView is a comprehensive medical technology platform facilitating seamless sharing of medical records, including diagnostic imaging exams. The customizable, white-label platform supports various medical use-cases, offering features such as secure video consultations, a 510k diagnostic DICOM image viewer, and medical reporting tools. MDView also provides a direct-to-patient radiology second opinion service, connecting patients with expert radiologists for virtual second opinion reports and video consultations. For details or business inquiries, visit https://www.mdview.com.About Modern TeleradiologyModern Teleradiology is a physician owned and led teleradiology service focused on providing state-of-the-art care to patients and dedicated collaboration to clients by offering remote radiology solutions customized to meet coverage needs. https://modernteleradiology.com/ Media Contact:Tracy Amato914-497-9245[email protected]SOURCE MDView Management, LLC
Teleflex Awarded Peripheral Access Agreement with Premier, Inc.
WAYNE, Pa., Dec. 05, 2023 (GLOBE NEWSWIRE) — Teleflex Incorporated (NYSE: TFX), a leading global provider of medical technologies, today announced it was awarded the Peripheral Access purchasing agreement with Premier, Inc. Effective December 1st, 2023, the new agreement allows Premier members, at their discretion, to take advantage of special […]
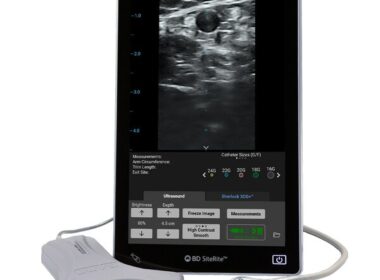
BD Launches Advanced Vascular Access Ultrasound System Designed to Improve Clinician Efficiency
SiteRite™ 9 Ultrasound System Delivers Enhanced Image Quality and Vessel Assessment Tools to Aid in First Attempt IV Insertion Success FRANKLIN LAKES, N.J., Nov. 30, 2023 /PRNewswire/ — BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today…
Wake Radiology UNC REX Healthcare Chooses Enlitic’s ENDEX to Augment Their Radiology Business
Wake Radiology is looking to increase the capacity of their radiology team with artificial intelligence and data standardization FORT COLLINS, Colo., Nov. 29, 2023 (GLOBE NEWSWIRE) — Enlitic®, Inc. and Wake Radiology UNC REX Healthcare will deploy the ENDEX™ data standardization toolset to help improve their reporting workflows and increase […]
Philips launches ultra lightweight and flexible MR Smart Fit coils to improve radiology productivity and diagnostic confidence at #RSNA23
Philips Smart Fit 1.5T shoulder coil in use November 28, 2023 Amsterdam, the Netherlands and Chicago, USA – Royal Philips (NYSE: PHG; AEX: PHIA), a global leader in health technology, today launched three new fit for purpose MR Smart Fit coils at #RSNA23. RF coils are an essential part of […]
Nuance Accelerates the Adoption of Generative AI for Radiology with PowerScribe Advanced Auto Impression Capability
Health systems adopting new copilot capabilities include Mass General Brigham and Quantum; early adopters cite time-savings and lower workload-related stress as key benefits of automated creation of draft radiology report impression and recommendation CHICAGO, Nov. 28, 2023 /PRNewswire/…