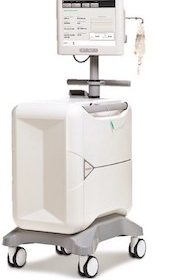
MONTREAL, June 7, 2019 /PRNewswire/ — Jubilant DraxImage Inc. (“DraxImage”), a wholly owned subsidiary of Jubilant Pharma Limited, is pleased to announce the receipt of the CE Mark certificate for its state-of-the-art RUBY Rubidium Elution System (RbES) and the proprietary RUBY consumable accessories dedicated for use with their RUBY-FILL®(Rubidium Rb82 Generator). This certificate allows the […]
Radiology
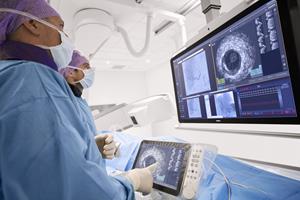
Philips launches new IntraSight interventional applications platform to seamlessly integrate intravascular imaging and physiology applications for image-guided procedures
Integrating best-in-class physiology, imaging and co-registration tools across the interventional lab workflow supports physicians in providing cardiac and peripheral vascular patients with superior care Amsterdam, The Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced the launch of the new IntraSight interventional applications platform. The secure, application-based […]
Embolx Extends the Sniper Balloon Occlusion Microcatheter Family with Launch of New K-Tip Design
SUNNYVALE, Calif.–(BUSINESS WIRE)–Embolx, Inc., a medical device company developing advanced microcatheters for arterial embolization procedures, today announced the commercial availability of the new Sniper® K™-tip in the United States and Europe. The Sniper Balloon Occlusion family of microcatheters now includes the new K-tip, a 45-degree angled tip designed to enhance […]
Fujifilm To Showcase Full Suite Of Specialized Pediatric Imaging Solutions At SPR 2019
LEXINGTON, Mass., April 25, 2019 /PRNewswire/ — (Booth 33/34) – FUJIFILM Medical Systems U.S.A., Inc., a leading provider of diagnostic imaging and medical informatics solutions, will present its comprehensive portfolio of pediatric solutions, including digital radiography (DR) and healthcare information technology (IT), at the Society for Pediatric Radiology Annual Meeting (SPR). The event, which is dedicated to fostering excellence […]
UC Davis Health Images First U.S. Patient Using Ultra-High Resolution CT with Canon Medical’s Aquilion Precision
TUSTIN, Calif.–(BUSINESS WIRE)–University of California, Davis (UC Davis) Health is the first institution in the U.S. to use Ultra-High Resolution CT (UHR CT) for clinical imaging, thanks to the installation of the Aquilion Precision™ from Canon Medical Systems USA, Inc. Reports in the initial use of this technology from global centers indicate significantly […]
Fujifilm Presents Full Solutions for Medical Image Generation, Management, Storage, and Analysis at SAGES 2019
VALHALLA, N.Y., March 28, 2019 /PRNewswire/ — (Booth #629) – Fujifilm will be premiering its innovative solutions for medical image generation, management, storage, and analysis, during the Society of American Gastrointestinal and Endoscopic Surgeons 2019 Annual Meeting (SAGES 2019) from April 3-6, 2019 at the Baltimore Convention Center in Baltimore, Maryland. Featured at the event will be FUJIFILM New Development, U.S.A., […]
Genetesis CardioFlux™ Platform Receives FDA 510(k) Clearance
MASON, Ohio–(BUSINESS WIRE)–Genetesis, a medical technology company focused on using biomagnetic imaging to enable rapid, noninvasive and accurate chest pain triage, has received FDA 510(k) clearance for its cardiac imaging platform. The platform pairs the CardioFlux™ Magnetocardiograph with the integrated Faraday Analytical Cloud™ (FAC). “This milestone provides emergency room physicians […]
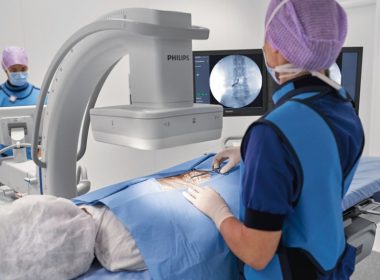
Philips launches Zenition mobile C-arm platform for enhanced operating room performance and workflow efficiency
AMSTERDAM, Feb. 18, 2019 /PRNewswire/ — Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced the launch of Philips Zenition, its new mobile C-arm imaging platform. Mobile C-arms are X-ray systems that are brought into the operating room (OR) to provide live image guidance during a wide range of surgeries including […]
iSchemaView Introduces New Mobile and Notification Platform Features Across Its Family of RAPID Products
MENLO PARK, Calif.–(BUSINESS WIRE)–iSchemaView, the worldwide leader in advanced imaging for stroke, today announced significant upgrades to the RAPID APP as well as other messaging and notification capabilities. In its latest release, the world’s most powerful and feature-rich neuroimaging software, RAPID, now includes additional customization of notifications for hospitals and […]
World’s First MRIdian Center Celebrates Five Years of MRI-Guided Radiation Treatments
CLEVELAND, Jan. 22, 2019 /PRNewswire/ — ViewRay, Inc. (Nasdaq: VRAY) announced today the five-year anniversary of patient treatments with MRIdian MRI-guided radiation therapy at Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine in St. Louis, Missouri. As one of the world’s leading cancer centers, Siteman is known for pioneering the advancement and adoption of […]