Clinically driven AI platform extends physicians’ ability to prioritize patients, reduce time to treatment and improve patient outcomes SAN MATEO, Calif.–(BUSINESS WIRE)–RapidAI, the global leader in neurovascular and vascular AI-enhanced clinical decision support and patient workflow, announced today that it […]
Regulatory
United Therapeutics Announces FDA Approval of Tyvaso DPI™
First approval of a dry powder inhaler for treatment of PAH and PH-ILD DPI device represents a convenient option for administration of treprostinil therapy Commercial launch activities underway; patient availability expected in June 2022 SILVER SPRING, Md. & RESEARCH TRIANGLE […]
VenoStent Technology Receives Breakthrough Device Designation by FDA
HOUSTON, TX / ACCESSWIRE / May 17, 2022 / VenoStent, Inc., a clinical-stage tissue engineering company developing bioabsorbable perivascular wraps to improve outcomes in the 5 million vascular surgeries performed each year, announces that The Center for Devices and Radiological Health […]
scPharmaceuticals Inc. Announces FDA Acceptance of FUROSCIX® New Drug Application
PDUFA action date set for October 8, 2022 Company preparing for Q4 commercial launch, if approved BURLINGTON, Mass., May 16, 2022 (GLOBE NEWSWIRE) — scPharmaceuticals Inc. (Nasdaq: SCPH), a pharmaceutical company focused on developing and commercializing products that have the […]
Vesalio Announces Completion of Enrollment in it’s FDA Enabling Stroke Study
Nashville, TN (May 11, 2022) – Vesalio is excited to announce the completion of patient enrollment for the CLEAR1 FDA IDE study utilizing the NeVa™ thrombectomy platform. This is an important milestone for Vesalio to complete for entering the U.S. neurovascular market […]
Medtronic receives FDA approval for latest generation drug-eluting coronary stent system
The Onyx Frontier™ drug-eluting stent offers an innovative delivery system and builds upon the acute performance and clinical data from the Resolute Onyx™ drug-eluting stent DUBLIN, May 13, 2022 /PRNewswire/ — Medtronic plc (NYSE:MDT), a global leader in healthcare technology, today announced it received […]
CVRx® Receives MR-Conditional Labeling Approval for its Barostim™ Heart Failure System
Heart failure patients implanted with Barostim can now receive conditional MRI scans MINNEAPOLIS, May 09, 2022 (GLOBE NEWSWIRE) — CVRx, Inc. (NASDAQ: CVRX) (“CVRx”), developer of the world’s first FDA-approved neuromodulation device to treat the symptoms of heart failure, has received […]
Acer Therapeutics Announces Agreement with FDA on Special Protocol Assessment for its Phase 3 EDSIVO™ (celiprolol) Trial in Vascular Ehlers-Danlos Syndrome Patients
Phase 3 DiSCOVER clinical trial initiation expected by end of Q2 2022 NEWTON, Mass., May 09, 2022 (GLOBE NEWSWIRE) — Acer Therapeutics Inc. (Nasdaq: ACER), a pharmaceutical company focused on the acquisition, development and commercialization of therapies for serious rare […]
Access Vascular Receives FDA Clearance for HydroPICC® Biomaterial-Based Dual-Lumen Catheter
Single- and dual-lumen biomaterial catheters allow hospitals to reduce thrombosis and vascular access complications for majority of PICC applications BILLERICA, Mass., May 05, 2022 (GLOBE NEWSWIRE) — Access Vascular, Inc. (AVI) today announced that it has received U.S. Food and […]
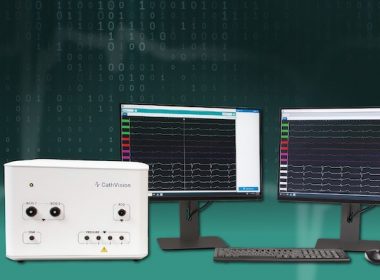
CathVision Announces FDA Clearance of ECGenius™ High-Fidelity, Low-Noise EP Recording Technology
ECGenius Sets a New Standard for ECG Signal Detection, Interpretation and Therapy Support COPENHAGEN, Denmark , May 4, 2022 /PRNewswire/ — CathVision, a medical technology company developing innovative electrophysiology solutions designed to enhance clinical decision making in the EP lab, today announced the FDA […]