SHANGHAI, July 15, 2022 /PRNewswire/ — Pulnovo Medical Limited, a globally recognized device pioneer in the treatment for cardiopulmonary diseases, today announced that it has successfully concluded the first Pre-Sub meeting with the U.S. Food and Drug Administration (“FDA”) for its Pulmonary Artery Denervation (PADN) Global Trial. Dr. Shaoliang Chen, Chair of Pulnovo […]
Regulatory
Ancora Heart Receives Breakthrough Device Designation from FDA for the AccuCinch® Ventricular Restoration System
Designation Allows for Expedited Review of Transcatheter Therapy Designed to Improve Left Ventricular Structure and Function in Heart Failure Patients with Reduced Ejection Fraction SANTA CLARA, Calif.–(BUSINESS WIRE)–Ancora Heart, Inc., a company developing a novel device-based therapy to address heart failure, today announced that the U.S. Food and Drug Administration […]
FDA Clears Eko’s Heart Disease Detection AI for Adults and Pediatrics
First and only machine learning algorithm to assist healthcare professionals in identifying structural heart murmurs using a smart stethoscope OAKLAND, Calif., July 12, 2022 /PRNewswire/ — Eko, a digital health company advancing heart and lung disease detection, today announced that the U.S. Food and Drug Administration (FDA) has cleared its Eko Murmur Analysis […]
Anthos Therapeutics Announces that Abelacimab has Received FDA Fast Track Designation for the Treatment of Thrombosis Associated with Cancer
Abelacimab is a dual-acting fully human monoclonal antibody targeting both Factor XI and Factor XIa with high affinity and selectivity Earlier this year abelacimab became the first-ever Factor XI inhibitor to begin enrolling patients in a Phase 3 trial (The ASTER Study) The AZALEA-TIMI 71 trial in patients with atrial fibrillation […]
Ra Medical Systems Receives FDA 510(k) Clearance for the DABRA 2.0 Catheter
Board of Directors continues its review of strategic alternatives to determine the Company’s optimal path forward CARLSBAD, Calif.–(BUSINESS WIRE)–Ra Medical Systems, Inc. (NYSE American: RMED) (“Ra Medical” or the “Company”), a medical device company focusing on developing its excimer laser system to treat vascular diseases, announces receipt of U.S. Food and […]
LIVEMETRIC RECEIVES FDA CLEARANCE FOR ITS WATCH-LIKE WEARABLE BLOOD PRESSURE MONITORING TECHNOLOGY, A LONG-AWAITED REVOLUTION IN CUFF-FREE HYPERTENSION MONITORING
LiveMetric is launching LiveOne, an FDA-cleared wearable technology for health systems, health insurers, and self-insured employers to improve the care and treatment of people with hypertension and cardiovascular disease. DENVER and LUXEMBOURG, June 30, 2022 /PRNewswire/ — LiveMetric, a leader in medical wearable technology, today announced the launch of LiveOne, the world’s first 510(k) U.S. Food and Drug Administration […]
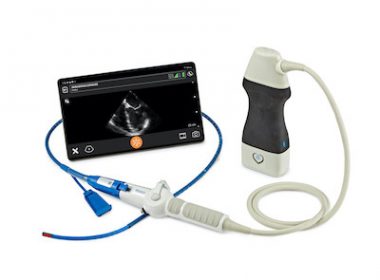
Clarius Introduces Partnership with ImaCor That Powers New FDA Cleared Handheld Hemodynamic Ultrasound
Now made ultra-portable with Clarius, ImaCor’s Zura Handheld Hemodynamic Ultrasound™ has received FDA clearance as the world’s first handheld transesophageal echocardiography (TEE) system, enabling more clinicians to accurately determine appropriate interventions in real-time. VANCOUVER, BC and JERICHO, N.Y., June 29, 2022 /PRNewswire/ — Clarius Mobile Health, a leading provider of high-definition wireless ultrasound systems, and ImaCor Inc, […]
Acutus Medical Announces FDA Clearance, Commercial Launch of AcQCross™ Line Extension Compatible with Watchman™ Delivery System
Innovative Transseptal Access System Is First and Only to Feature Integrated Dilator and Needle to Reduce Exchanges CARLSBAD, Calif., June 27, 2022 (GLOBE NEWSWIRE) — Acutus Medical, Inc. (“Acutus”) (Nasdaq: AFIB), an arrhythmia management company focused on improving the way cardiac arrhythmias are diagnosed and treated, today announced the commercial […]
SoniVie receives IDE approval from FDA for its Pilot study to treat Hypertension with its Renal Artery Denervation TIVUS™ technology
TEL AVIV, Israel, June 20, 2022 /PRNewswire/ — SoniVie, an Israeli company developing a novel proprietary Therapeutic Intra-Vascular Ultrasound System (TIVUS™) to treat a variety of hypertensive disorders, announced that on May 5th 2022 the U.S. Food and Drug Administration granted IDE approval for its “REDUCED1” Pilot study to treat Resistant Hypertension Patients with Renal […]
MEDICALGORITHMICS secured FDA approval for Qpatch wearable
The US Food and Drug Administration has registered Qpatch, an ECG wearable unit developed by experts from Medicalgorithmics. The FDA approval allows use of ECG readings from Qpatch in the United States. WARSAW, Poland, June 9, 2022 /PRNewswire/ — Qpatch is a state-of-the-art wearable device for measuring individual ECG signal to obtain accurate […]