HOUSTON, TX / ACCESSWIRE / May 17, 2022 / VenoStent, Inc., a clinical-stage tissue engineering company developing bioabsorbable perivascular wraps to improve outcomes in the 5 million vascular surgeries performed each year, announces that The Center for Devices and Radiological Health (CDRH) at the Food and Drug Administration (FDA) has granted […]
Regulatory
scPharmaceuticals Inc. Announces FDA Acceptance of FUROSCIX® New Drug Application
PDUFA action date set for October 8, 2022 Company preparing for Q4 commercial launch, if approved BURLINGTON, Mass., May 16, 2022 (GLOBE NEWSWIRE) — scPharmaceuticals Inc. (Nasdaq: SCPH), a pharmaceutical company focused on developing and commercializing products that have the potential to optimize the delivery of infused therapies, advance patient […]
Vesalio Announces Completion of Enrollment in it’s FDA Enabling Stroke Study
Nashville, TN (May 11, 2022) – Vesalio is excited to announce the completion of patient enrollment for the CLEAR1 FDA IDE study utilizing the NeVa™ thrombectomy platform. This is an important milestone for Vesalio to complete for entering the U.S. neurovascular market to treat patients suffering from acute ischemic stroke (AIS). AIS […]
Medtronic receives FDA approval for latest generation drug-eluting coronary stent system
The Onyx Frontier™ drug-eluting stent offers an innovative delivery system and builds upon the acute performance and clinical data from the Resolute Onyx™ drug-eluting stent DUBLIN, May 13, 2022 /PRNewswire/ — Medtronic plc (NYSE:MDT), a global leader in healthcare technology, today announced it received U.S. Food and Drug Administration (FDA) approval for the Onyx […]
CVRx® Receives MR-Conditional Labeling Approval for its Barostim™ Heart Failure System
Heart failure patients implanted with Barostim can now receive conditional MRI scans MINNEAPOLIS, May 09, 2022 (GLOBE NEWSWIRE) — CVRx, Inc. (NASDAQ: CVRX) (“CVRx”), developer of the world’s first FDA-approved neuromodulation device to treat the symptoms of heart failure, has received U.S. Food and Drug Administration (FDA) approval for magnetic resonance […]
Acer Therapeutics Announces Agreement with FDA on Special Protocol Assessment for its Phase 3 EDSIVO™ (celiprolol) Trial in Vascular Ehlers-Danlos Syndrome Patients
Phase 3 DiSCOVER clinical trial initiation expected by end of Q2 2022 NEWTON, Mass., May 09, 2022 (GLOBE NEWSWIRE) — Acer Therapeutics Inc. (Nasdaq: ACER), a pharmaceutical company focused on the acquisition, development and commercialization of therapies for serious rare and life-threatening diseases with significant unmet medical needs, today announced […]
Access Vascular Receives FDA Clearance for HydroPICC® Biomaterial-Based Dual-Lumen Catheter
Single- and dual-lumen biomaterial catheters allow hospitals to reduce thrombosis and vascular access complications for majority of PICC applications BILLERICA, Mass., May 05, 2022 (GLOBE NEWSWIRE) — Access Vascular, Inc. (AVI) today announced that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its HydroPICC® Dual-Lumen catheter. Designed […]
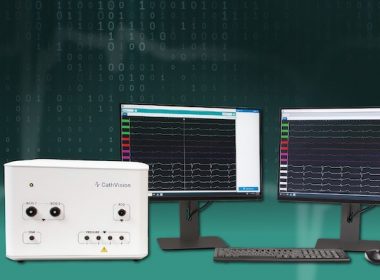
CathVision Announces FDA Clearance of ECGenius™ High-Fidelity, Low-Noise EP Recording Technology
ECGenius Sets a New Standard for ECG Signal Detection, Interpretation and Therapy Support COPENHAGEN, Denmark , May 4, 2022 /PRNewswire/ — CathVision, a medical technology company developing innovative electrophysiology solutions designed to enhance clinical decision making in the EP lab, today announced the FDA 510(k) clearance of the ECGenius EP Recording System. ECGenius, the […]
Silk Road Medical Announces FDA Approval of Expanded Indications for the ENROUTE® Transcarotid Stent System
SUNNYVALE, Calif., May 02, 2022 (GLOBE NEWSWIRE) — Silk Road Medical, Inc. (Nasdaq: SILK), a company focused on reducing the risk of stroke and its devastating impact, today announced that that the U.S. Food and Drug Administration (FDA) approved expanded indications for the ENROUTE stent to include patients at standard […]
Boston Scientific Receives FDA Clearance for the EMBOLD™ Fibered Detachable Coil
MARLBOROUGH, Mass., April 28, 2022 – Boston Scientific Corporation (NYSE: BSX) has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the EMBOLD™ Fibered Detachable Coil, which is indicated to obstruct or reduce the rate of blood flow in the peripheral vasculature. The first procedure using the device was performed this week […]