Camzyos is the first and only FDA-approved cardiac myosin inhibitor that specifically targets the source of obstructive HCM Approval based on groundbreaking Phase 3 EXPLORER-HCM trial demonstrating benefit in patients receiving Camzyos versus placebo PRINCETON, N.J.–(BUSINESS WIRE)–Bristol Myers Squibb (NYSE: BMY) today announced that the U.S. Food and Drug Administration (FDA) […]
Regulatory
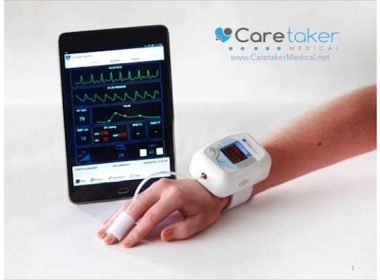
FDA Clears Caretaker Medical’s Wireless Monitor for NonInvasive & Continuous Cardiac Output, Stroke Volume, and Advanced Hemodynamics
VitalStream™, Featuring Patented Pulse Decomposition Analysis™ technology becomes the World’s First Wireless Wearable Monitor for Continuous Blood Pressure and Advanced Hemodynamics – All with a single, easy-to-apply Finger Sensor Caretaker Medical, a digital health leader in continuous “beat by beat” wireless patient monitoring technologies, today announced that it has received […]
TransMedics Receives FDA PMA Approval of OCS™ DCD Heart Indication
ANDOVER, Mass., April 28, 2022 /PRNewswire/ — TransMedics Group, Inc. (“TransMedics”) (Nasdaq: TMDX), a medical technology company that is transforming organ transplant therapy for patients with end-stage lung, heart, and liver failure, today announced that the U.S. Food and Drug Administration (FDA) has granted premarket approval (PMA) of its OCS™ Heart System for […]
BIOTRONIK Announces FDA Approval of Renamic Neo with LiveSupport Software
LAKE OSWEGO, Ore., April 26, 2022 /PRNewswire/ — BIOTRONIK, Inc. announced today it has received U.S. Food and Drug Administration (FDA) approval of Renamic Neo, the company’s state-of-the-art programmer for implanted cardiac rhythm management devices such as ICDs, pacemakers, and implantable cardiac monitors. In addition to its programming functions, Renamic Neo now […]
FDA Clearance Granted for New Dual Lumen Catheter That Enables Precise Visualization and Treatment Access to the Left Heart
SCOTTSDALE, Ariz.–(BUSINESS WIRE)–Franklin Mountain Medical announced today that the company has received FDA clearance of its patented UltraNav Transseptal Catheter System for controlled access and delivery of cardiovascular catheters and guidewires to the heart chambers via transseptal puncture. The success of transseptal procedures depends on the clinician’s ability to adequately […]
BENDIT Technologies Receives U.S. Food and Drug Administration 510(k) Clearance of its Bendit 021″ steerable microcatheter
TEL AVIV, Israel, April 18, 2022 /PRNewswire/ — BENDIT Technologies, a company focused on the development of a steerable microcatheter platform, announced today that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Bendit21 microcatheter for treatment in the neuro, peripheral, and coronary vasculature. The clearance was received several […]
FDA Clears Four New Coronary Micro Support Catheters
Non-tapered metal-alloy support catheter platform receives FDA clearance for use in coronary and peripheral vasculature. Transit Scientific now has one of the largest platforms of FDA cleared microcatheters in the industry. PARK CITY, Utah, April 12, 2022 /PRNewswire/ — Transit Scientific announced the FDA clearance of its XO Cross® Support Catheter Platform to […]
TRUVIC Announces 510(k) Clearance for the Prodigy™ Thrombectomy System
First regulatory milestone as part of a strategy to advance patient care CAMPBELL, Calif.–(BUSINESS WIRE)–Truvic Medical, Inc., a wholly owned subsidiary of Imperative Care, Inc., today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the Prodigy™ Thrombectomy System, designed for the treatment of peripheral […]
Israeli startup Sanolla receives FDA clearance for the world’s first AI-ready infrasound stethoscope
Sanolla’s pioneering technology draws lifesaving medical insights from listening to bodily sounds that cannot be heard by humans The startup’s AI algorithms provide unmatched disease classification for many cardiopulmonary diseases including COPD, pneumonia, asthma, and cardiac morbidities NESHER, Israel, April 11, 2022 /PRNewswire/ — Israeli startup Sanolla, which provides AI-powered primary care diagnostic solutions, announced […]
Triastek Receives FDA IND Clearance for 3D Printed Product of Blockbuster Molecule
NANJING, China, April 7, 2022 /PRNewswire/ — Triastek, Inc. (“Triastek”) recently announced that the United States Food and Drug Administration (FDA) has granted permission to begin clinical studies of its Investigational New Drug (IND) 505(b)(2) application for a 3D printed drug product – T20. It is Triastek’s second product receiving IND clearance from the […]