Receives eighth FDA clearance, releases its next-generation Cardio AI application SAN FRANCISCO, April 07, 2022 (GLOBE NEWSWIRE) — Arterys, the world’s leading vendor-neutral AI platform, has announced several new modules to its already robust Cardio AI clinical application and an additional FDA AI clearance based on deep learning. Cardio AI […]
Regulatory
Zio® by iRhythm Shown to Prevent Hospital Admissions, Aid in Early Detection of AF
New clinical research presented at ACC.22 shows that the Zio service is a viable solution for the early detection of silent atrial fibrillation Additional data shows that Zio AT can positively impact hospital resources – saving one healthcare system 136 inpatient hospitalization days SAN FRANCISCO, April 03, 2022 (GLOBE NEWSWIRE) — iRhythm […]
ABBOTT RECEIVES FDA APPROVAL FOR AVEIR™ VR LEADLESS PACEMAKER SYSTEM TO TREAT PATIENTS WITH SLOW HEART RHYTHMS
Abbott’s Aveir single chamber (VR) pacing system is the world’s only leadless pacemaker with a unique mapping capability to assess correct positioning prior to placement Aveir VR has an increased projected battery life that can be up to two times longer than other commercially available leadless pacemakers when using the […]
Aziyo Announces FDA 510(k) Submission for CanGaroo® RM, its Next-Generation Biomaterial Envelope Enhanced with Antibiotics
SILVER SPRING, Md., April 04, 2022 (GLOBE NEWSWIRE) — Aziyo Biologics, Inc. (Nasdaq: AZYO), today announced the Company has filed a 510(k) premarket notification to the U.S. Food and Drug Administration (FDA) for CanGaroo® RM Antibacterial Envelope, its next-generation biomaterial envelope for use with implantable electronic devices (IED). Aziyo Biologics is […]
FEops HEARTguide Receives FDA Clearance for LAAo Planning Capabilities
GENT, Belgium–(BUSINESS WIRE)–FEops today announced that it received authorization from the U.S. Food and Drug Administration (FDA) for FEops HEARTguide™ pre-operative planning of left atrial appendage occlusion (LAAo) with the Abbott’s Amplatzer™ Amulet™ and Boston Scientific’s Watchman FLX™ device*. FEops HEARTguide is a one-in-its-kind cloud-based procedure planning solution in the structural […]
Radiaction Medical Ltd. Receives FDA Clearance for its Innovative Radiation Protection System and Secures $10M for US Launch and Commercialization
TEL AVIV, Israel, March 31, 2022 /PRNewswire/ — Radiaction Medical Ltd. (“Radiaction”), an innovative medical device company focused on radiation protection in the interventional cardiology and electrophysiology sectors, announced FDA 510(K) clearance for the marketing of its Shield System in the US. In addition, the company has completed a $10 million round of financing led by […]
EDWARDS MITRIS RESILIA VALVE RECEIVES FDA APPROVAL FOR MITRAL REPLACEMENT SURGERIES
IRVINE, Calif., March 31, 2022 /PRNewswire/ — Edwards Lifesciences (NYSE: EW) today announced it received approval from the U.S. Food and Drug Administration (FDA) for the MITRIS RESILIA valve, a tissue valve replacement specifically designed for the heart’s mitral position. The MITRIS RESILIA valve has a saddle-shaped sewing cuff that mimics the asymmetric […]
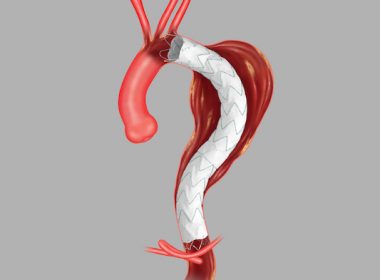
Cook Medical receives FDA Breakthrough Device Designation for Zenith® Thoraco+ Endovascular System
Bloomington, Ind. — Cook Medical’s Zenith® Thoraco+ Endovascular System (Thoraco+) has received Breakthrough Device Designation from the US Food and Drug Administration (FDA). This designation is granted to devices that have the potential to provide more effective treatment or diagnosis for life-threatening or irreversibly debilitating diseases or conditions. While the product is […]
FDA Clears the Biobeat Remote Patient Monitoring Device and Platform for Additional Vital Signs
With Respiratory Rate and Body Temperature Now Cleared, Biobeat is Leading the Medical Grade Remote Patient Monitoring Market PETAH TIKVA, Israel, March 28, 2022 /PRNewswire/ — Biobeat, a global leader in wearable remote patient monitoring solutions for the healthcare continuum, announced today that its wearable remote patient monitoring device have received 510(k) clearance from […]
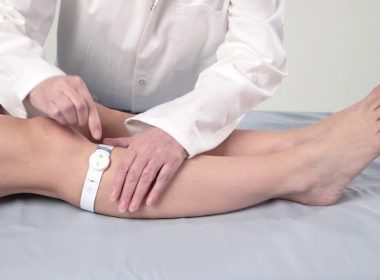
Sky Medical Technology wins further FDA clearance to market the new (W3) geko™ device variant for venous insufficiency and ischemia
DARESBURY, England, March 25, 2022 /PRNewswire/ –Sky Medical Technology Ltd (Sky) has today announced it has achieved further U.S. Food and Drug Administration (FDA) 510(k) clearance to market the new (W3) geko™ device variant, for increasing microcirculatory blood flow in lower limb soft tissue of patients with venous insufficiency and/or ischemia. This latest […]