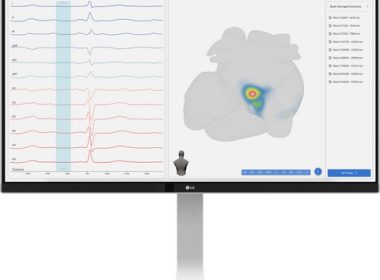
SAN DIEGO–(BUSINESS WIRE)–Vektor Medical, a medical technology company transforming cardiac arrhythmia care, announced it has received CE Mark for the vMap® System, a non-invasive tool developed with AI that transforms standard 12-lead ECG data into 3D arrhythmia source maps in under a minute. “vMap is emerging as a simple, non-invasive […]
Regulatory
Lexeo Therapeutics Announces Progress in FDA Discussions for Accelerated Approval Pathway and Positive Interim Clinical Data for LX2006 in Friedreich Ataxia Cardiomyopathy
U.S. Food and Drug Administration (FDA) open to pooling data from ongoing Phase I/II studies of LX2006 with pivotal data to support a Biologics License Application (BLA) for Accelerated Approval Interim clinical data show sustained or deepening improvements in the majority of participants across both cardiac and neurologic measures of Friedreich ataxia Participants with abnormal left ventricular mass index (LVMI) at baseline achieved 18% mean reduction in LVMI at 6 months and 23% mean reduction at 12 months, exceeding FDA-aligned target threshold of 10% reduction Clinically meaningful improvement observed in the modified Friedreich Ataxia Rating Scale (mFARS), indicative of slowed disease progression and improved function Company to host webcast today at 8:00 AM ET NEW YORK, Oct. 07, 2025 (GLOBE NEWSWIRE) — Lexeo Therapeutics, Inc. (Nasdaq: LXEO), a clinical stage genetic medicine company dedicated to pioneering novel treatments for cardiovascular diseases, today announced updates to key components of an Accelerated Approval pathway for LX2006 in Friedreich ataxia (FA) cardiomyopathy, alongside new interim clinical data from ongoing Phase I/II studies. “We are encouraged by our recent dialogue with the FDA on LX2006, and we appreciate the Agency’s collaborative spirit as we work to deliver a potentially life-changing therapy to the FA community as efficiently as possible,” said R. Nolan Townsend, Chief Executive Officer of Lexeo Therapeutics. “Given the highly compelling data to date that demonstrate clinically meaningful improvements across both cardiac and neurologic measures of FA, we are now pursuing a development strategy that could enable a smaller pivotal study, given the potential to pool data with the ongoing Phase I/II trials, as well as potentially assessing the co-primary endpoint of LVMI earlier than 12 months. This approach could accelerate our overall timeline toward a BLA submission for LX2006 under the Accelerated Approval pathway.” FDA Feedback to DateIn response to questions from the Company regarding the possibility of a faster path to a BLA, the FDA has indicated openness to a BLA submission for accelerated approval that includes clinical data from the ongoing Phase I/II studies of LX2006 pooled with new clinical data to be generated in the planned pivotal study. To enable pooling of these data to support licensure, Lexeo will submit enhanced manufacturing comparability data and meet an additional nonclinical requirement prior to the initiation of the planned pivotal study, given the Company’s intention to leverage its optimized, high-yield Sf9-baculovirus manufacturing platform for future clinical and commercial drug supply, compared to the adherent HEK293 process used for Phase I/II clinical supply. The FDA also previously agreed to evaluate the co-primary endpoint of LVMI at a time point earlier than 12 months. Lexeo continues to engage with the FDA on the pivotal protocol and comparability. In discussions to date, there have been no changes to the previously disclosed alignment with the FDA on key parameters related to the LX2006 planned registrational study. Collectively, Lexeo believes this regulatory feedback has the potential to reduce the size and length of the planned pivotal study, possibly accelerating the overall timeline to BLA submission. Lexeo plans to initiate the LX2006 pivotal study as soon as possible in the first half of 2026, pending finalization of the trial protocol. FDA has previously granted Breakthrough Therapy, Regenerative Medicine Advanced Therapy (RMAT), Orphan Drug and Fast Track designations to LX2006, and admitted LX2006 into the CMC Development and Readiness Pilot (CDRP) program. LX2006 Interim Clinical Update (n=16 participants with >6-months of follow-up)Updated interim clinical data from both ongoing Phase I/II studies of LX2006 continue to show encouraging safety and efficacy, exceeding the thresholds previously agreed with FDA for co-primary endpoints LVMI and frataxin expression. Left ventricular mass index (LVMI): Among participants with abnormal baseline LVMI (key inclusion criteria for pivotal study; n=6): 6 of 6 participants achieved LVMI measurements within the normal range as of latest visit5 of 6 participants achieved >10% improvement by 12 months18% mean improvement in LVMI at 6 months and 23% mean improvement at 12 months, exceeding 10% FDA-aligned threshold for pivotal study28% mean improvement in LVMI at 6 months and 33% mean improvement at 12 months in mid- and high-dose cohorts (n=3), suggesting dose-dependent improvement Among participants with normal baseline LVMI (n=10), the majority demonstrated LVMI improvement or stabilization over time Secondary cardiac biomarkers: 14 of 16 participants achieved >25% reduction in high-sensitivity troponin I from baseline at latest visit14 of 16 participants achieved reduction or stabilization in lateral wall thickness (LWT) from baseline at latest visit Modified Friedreich Ataxia Rating Scale (mFARS): 2.0-point mean improvement from baseline at latest visit across all participants with >6-months of follow-up (n=16)11 of 16 participants achieved reduction or stabilization in mFARS from baseline at latest visit Previously reported data from Lexeo’s ongoing SUNRISE-FA trial (n=8) showed that all study participants achieved increases in frataxin protein expression from baseline at 3 months, with dose-dependent increases observed across cohorts. LX2006 Interim Safety Update (n=17 participants) Treatment with LX2006 has been generally well tolerated with no Grade 3+ SAEs to dateNo clinically significant complement activationMinimal, transient liver function test (LFT) elevationsNo signs of frataxin over-expression observed in cardiac tissueNo participants discontinued from either studyOne previously disclosed, possibly treatment-related Grade 2 event of asymptomatic myocarditis observed one year after dosing Corporate Webcast DetailsLexeo Therapeutics will host a webcast at 8:00 AM ET today, October 7, 2025. Analysts and investors can participate by accessing the webcast live on the News & Events page in the Investors section of Lexeo’s website, www.lexeotx.com. The webcast will be archived on the company’s website following completion of the call, and presentation materials will be available on the Presentations page of the website. About LX2006LX2006 is an AAV-based gene therapy candidate for the treatment of FA cardiomyopathy, the leading cause of death in individuals with FA which affects approximately 5,000 people in the United States. LX2006 is designed to systemically deliver a functional frataxin gene to promote the expression of the frataxin protein and restore mitochondrial function in myocardial cells. LX2006 is being evaluated in two clinical trials, the Lexeo-sponsored SUNRISE-FA Phase 1/2 clinical trial (NCT05445323) and the Weill Cornell Medicine investigator-initiated Phase 1A trial (NCT05302271). LX2006 has been granted Breakthrough Therapy, Regenerative Medicine Advanced Therapy, Orphan Drug, Rare Pediatric Disease and Fast Track designations by the FDA, admitted into the FDA CMC Development and Readiness Pilot (CDRP) program, and granted orphan medicinal product designation by the European Commission. About Lexeo TherapeuticsLexeo Therapeutics is a New York City-based, clinical stage genetic medicine company dedicated to reshaping heart health by applying pioneering science to fundamentally change how cardiovascular diseases are treated. The Company is advancing a portfolio of therapeutic candidates that take aim at the underlying genetic causes of conditions, including LX2006 in Friedreich ataxia (FA) cardiomyopathy, LX2020 in plakophilin-2 (PKP2) arrhythmogenic cardiomyopathy, and others in devastating diseases with high unmet need. Cautionary Note Regarding Forward-Looking StatementsCertain statements in this press release may constitute “forward-looking statements” within the meaning of the federal securities laws, including, but not limited to, Lexeo’s expectations and plans regarding its current product candidates and programs, the structure of and timelines for completion of any current or additional clinical trials required by the FDA, the timing for receipt and announcement of data from any such clinical trials, and the timing and likelihood of potential regulatory developments, trial design changes and approval. Words such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “design,” “estimate,” “predict,” “potential,” “develop,” “plan” or the negative of these terms, and similar expressions, or statements regarding intent, belief, or current expectations, are forward-looking statements. While Lexeo believes these forward-looking statements are reasonable, undue reliance should not be placed on any such forward-looking statements. These forward-looking statements are based upon current information available to the company as well as certain estimates and assumptions and are subject to various risks and uncertainties (including, without limitation, those set forth in Lexeo’s filings with the U.S. Securities and Exchange Commission (SEC)), many of which are beyond the company’s control and subject to change. Actual results could be materially different from those indicated by such forward-looking statements as a result of many factors, including but not limited to: risks and uncertainties related to global macroeconomic conditions and related volatility; expectations regarding the initiation, progress, and expected results of Lexeo’s preclinical studies, clinical trials and research and development programs; the unpredictable relationship between preclinical study results and clinical study results; delays in submission of regulatory filings or failure to receive regulatory approval; liquidity and capital resources; and other risks and uncertainties identified in Lexeo’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2025, filed with the SEC on August 14, 2025, and subsequent future filings Lexeo may make with the SEC. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Lexeo claims the protection of the Safe Harbor contained in the Private Securities Litigation Reform Act of 1995 for forward-looking statements. Lexeo expressly disclaims any obligation to update or alter any statements whether as a result of new information, future events or otherwise, except as required by law. Media Response:Media@lexeotx.com Investor Response:Carlo Tanzi, Ph.D.ctanzi@kendallir.com
Daxor Unveils New FDA-Cleared BVA Analyzer Amid Strong Market Demand and Pivotal Clinical Data at HFSA
Three Studies Validate BVA-Guided Care Significantly Cuts Hospital Readmissions and Boosts Survival in High-Cost Heart Failure Segments
Pulnovo Medical Receives two IDE approvals for PADN system’s clinical trials with CMS coverage
SHANGHAI, Sept. 12, 2025 /PRNewswire/ — Pulnovo Medical, a globally recognized leader in medical devices for pulmonary hypertension (PH) and heart failure (HF), is proud to announce that its PADN catheter and generator has received two Investigational Device Exemption (IDE) approvals—HDE…
Microbot Medical® Receives FDA 510(k) Clearance for Its LIBERTY® Endovascular Robotic System
Microbot Medical® Receives FDA 510(k) Clearance for Its LIBERTY® Endovascular Robotic System.
Medtronic receives approval for Symplicity Spyral™ Renal Denervation System in Japan
Japan is now the 77th country to receive regulatory approval for the Symplicity Spyral Renal Denervation System Medtronic, a global leader in healthcare technology, today announced it received approval in Japan from the Pharmaceuticals and Medical Devices Agency (PMDA) for its Symplicity Spyral™ renal denervation (RDN) system, also known as […]
Catheter Precision Receives Approval for LockeT in the United Kingdom
FORT MILLS, S.C., Aug. 18, 2025 (GLOBE NEWSWIRE) — Catheter Precision, Inc. (VTAK – NYSE/American), a US based medical device company focused on developing technologically advanced products for the cardiac electrophysiology market announced registration and approval in the United Kingdom for its LockeT product, a suture retention device. LockeT received CE Mark for European approval and distribution in May 2025. Obtaining registration was the final approval required to launch sales in the UK.
Instylla Gains U.S. FDA Premarket Approval for Embrace™ Hydrogel Embolic System
First and Only Liquid Embolic Approved for Hypervascular Tumor Embolization Supported by a Prospective, Randomized, Controlled, Clinical Trial BEDFORD, Mass., Aug. 7, 2025 /PRNewswire/ — Instylla, Inc., a privately held company developing novel resorbable embolics for peripheral vascular…
Boston Scientific receives FDA approval for expanded labeling of FARAPULSE™ Pulsed Field Ablation System
The FARAPULSE PFA System now approved for pulmonary vein and posterior wall ablation in patients with persistent atrial fibrillation MARLBOROUGH, Mass., July 7, 2025 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) has received U.S. Food and Drug Administration (FDA) approval to…
InspireMD Announces CE Mark Approval for CGuard® Prime Embolic Prevention System (EPS) Under European MDR for the Prevention of Stroke
MIAMI, June 13, 2025 (GLOBE NEWSWIRE) — InspireMD, Inc. (Nasdaq: NSPR), developer of the CGuard® Prime carotid stent system for the prevention of stroke, today announced the company has received CE Mark approval under the European Medical Device Regulation (MDR) for the CGuard® Prime EPS.