Malmö, Sweden, February 9, 2022 Acarix submits breakthrough designation request with FDA for heart failure diagnosis Acarix expands its portfolio and submits a breakthrough designation request for its innovative technology for heart failure diagnosis with the Food and Drug Administration (FDA) in USA. Heart failure affects more than 6 million people in the USA at […]
Regulatory
atHeart Medical Receives FDA Approval for the Second Phase of the ASCENT ASD U.S. IDE Trial
Company’s reSept™ ASD Occluder Aims to Evolve Septal Closure with its Novel Metal-Free Frame Design BAAR, Switzerland and SANTA CLARA, Calif., Feb. 8, 2022 /PRNewswire/ — atHeart Medical, a medical device company dedicated to establishing the new standard of care for closure of atrial septal defects (ASD), today announced it has received approval for the start […]
Leqvio® Therapy to Lower Cholesterol Approved by FDA, IVX Health Now Accepting New Leqvio Patients
NASHVILLE, Tenn., Feb. 8, 2022 /PRNewswire/ — IVX Health, a national provider of infusion and injection therapy for patients with complex chronic conditions, announced today the expansion of its injection formulary with the addition of Leqvio® (inclisiran), a new FDA-approved therapy by Novartis Pharmaceuticals Corporation indicated for the treatment of adult patients with clinical atherosclerotic cardiovascular disease (ASCVD) […]
CereVasc Receives FDA IDE Approval to Begin Initial Clinical Study of the eShunt® System for Patients with Normal Pressure Hydrocephalus
BOSTON, Feb. 8, 2022 /PRNewswire/ — CereVasc, Inc., a privately held, clinical-stage, medical device company developing novel, minimally invasive treatments for neurological diseases, announced today that the U.S. Food and Drug Administration (FDA) has approved an investigational device exemption (IDE) application to initiate a pilot trial of the eShunt System in patients […]
Cytokinetics Announces FDA Acceptance of New Drug Application for Omecamtiv Mecarbil for the Treatment of Heart Failure With Reduced Ejection Fraction
PDUFA Target Action Date Set for November 30, 2022 FDA is Currently Not Planning to Hold an Advisory Committee Meeting to Discuss the Application SOUTH SAN FRANCISCO, Calif., Feb. 04, 2022 (GLOBE NEWSWIRE) — Cytokinetics, Incorporated (Nasdaq: CYTK) today announced that the U.S. Food & Drug Administration (FDA) has accepted and […]
BioCardia Receives FDA Breakthrough Device Designation for CardiAMP Cell Therapy System for Heart Failure
SUNNYVALE, Calif.–(BUSINESS WIRE)–BioCardia®, Inc. (Nasdaq: BCDA), a developer of cellular and cell-derived therapeutics for the treatment of cardiovascular and pulmonary diseases, today announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for the CardiAMP® Cell Therapy System for the treatment of heart failure. It is believed […]
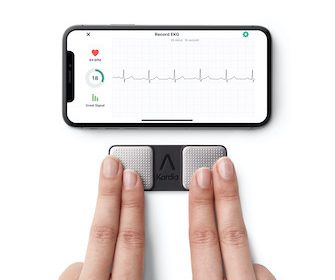
FDA Clears World’s First Credit-Card-Sized Personal ECG
AliveCor launches KardiaMobile Card, the only personal ECG slim enough to fit in a wallet for instant feedback on heart health anytime, anywhere MOUNTAIN VIEW, Calif., Feb. 1, 2022 /PRNewswire/ — AliveCor, the leading innovator in FDA-cleared personal electrocardiogram (ECG) technology, today announced the launch of KardiaMobile Card, the slimmest, most convenient personal […]
AIROS Medical and Fist Assist Devices, LLC Announce Launch of Commercial Website for Fist Assist FA-1 Wearable Medical Device
LAS VEGAS–(BUSINESS WIRE)–AIROS Medical, Inc., a designer, manufacturer, and distributor of compression therapy devices, and Fist Assist Devices, LLC (Fist Assist), an innovative medical device company, today announced the launch of an e-commerce website, www.fistassistusa.com, for the sale of the Fist Assist FA-1 compression device. The FDA 510(k)-cleared Fist Assist Model FA-1 […]
Imara Announces FDA Clearance of Investigational New Drug Application (IND) for Tovinontrine (IMR-687) for Heart Failure with Preserved Ejection Fraction (HFpEF)
Expanding patient base and potential of Imara’s small molecule oral inhibitor of phosphodiesterase-9 (PDE9) alongside hemoglobin disorders Phase 2 trial aims to select HFpEF patients with enriched PDE9 expression for targeted approach to a heterogeneous disease Study initiation planned for second quarter of 2022 BOSTON, Jan. 25, 2022 (GLOBE NEWSWIRE) […]
Eplontersen granted Orphan Drug Designation in the US for transthyretin amyloidosis
WILMINGTON, Del., January 24, 2022 – Eplontersen has been granted Orphan Drug Designation (ODD) in the US by the Food and Drug Administration (FDA) for the treatment of transthyretin-mediated amyloidosis, a systemic, progressive and fatal condition. Eplontersen, formerly known as IONIS-TTR-LRx, is a ligand-conjugated antisense (LICA) investigational medicine currently in […]