SOUTH SAN FRANCISCO, Calif.–(BUSINESS WIRE)–InterVene Inc. announced today that it has received Breakthrough Device Designation by the FDA for the company’s BlueLeaf® Endovenous Valve Formation System. BlueLeaf is the first catheter-based solution developed for deep vein reflux — the failure of venous valves in the legs — which does not require […]
Regulatory
Cytokinetics Announces Receipt of Breakthrough Therapy Designation from FDA for Aficamten
FDA Granted Designation for the Treatment of Symptomatic Obstructive Hypertrophic Cardiomyopathy Based on Results of REDWOOD-HCM SOUTH SAN FRANCISCO, Dec. 09, 2021 (GLOBE NEWSWIRE) — Cytokinetics, Incorporated (Nasdaq: CYTK) today announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Therapy Designation for aficamten for the treatment of symptomatic obstructive […]
Cardiologs’ AI Receives 510(k) Clearance for Pediatric Use
New data shows improved deep learning algorithm performs equally across all age groups, enabling more patients to benefit from the company’s cardiac diagnostics AI platform PARIS and BOSTON, Nov. 22, 2021 /PRNewswire/ — Cardiologs announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to use its AI-powered cardiac diagnostics platform […]
Avinger Announces FDA Clearance of Pantheris for the Treatment of In-Stent Restenosis
New indication expands addressable market for Pantheris REDWOOD CITY, CA / ACCESSWIRE / November 17, 2021 / Avinger, Inc. (NASDAQ:AVGR), a commercial-stage medical device company marketing the first and only intravascular image-guided, catheter-based system for diagnosis and treatment of Peripheral Artery Disease (PAD), today announced that it has received 510(k) clearance […]
Bristol Myers Squibb Announces New PDUFA Date for Mavacamten
PRINCETON, N.J.–(BUSINESS WIRE)–Bristol Myers Squibb (NYSE: BMY) today announced that the U.S. Food and Drug Administration (FDA) has extended the review of the New Drug Application (NDA) for mavacamten for the treatment of patients with symptomatic obstructive hypertrophic cardiomyopathy (oHCM) to April 28, 2022. The FDA notified Bristol Myers Squibb on […]
Cardialen Receives FDA Approval to Conduct Clinical Trial of Low-Energy Atrial Fibrillation Treatment
U.S. Food and Drug Administration approval of a clinical trial of Cardialen’s low-energy defibrillation and cardioversion therapy expands the number of leading clinical institutions evaluating its promising MultiPulse™ Therapy. MINNEAPOLIS–(BUSINESS WIRE)–Cardialen, Inc., has received approval from the U.S. Food and Drug Administration (FDA) for an Investigational Device Exemption (IDE) to begin a […]
US FDA accepts supplemental New Drug Application and grants Priority Review for Jardiance® for adults with heart failure independent of left ventricular ejection fraction
If approved, Jardiance would be the first clinically proven treatment for adults across the full spectrum of heart failure regardless of ejection fraction RIDGEFIELD, Conn. and INDIANAPOLIS, Nov. 11, 2021 /PRNewswire/ — The U.S. Food and Drug Administration (FDA) has accepted a supplemental New Drug Application (sNDA) and granted Priority Review for Jardiance® (empagliflozin) 10 […]
HeartVista Receives FDA 510(k) Clearance to Deliver One Click MRI™ on Siemens Healthineers MRI Scanners
– FDA 510(k) Clearance Creates Multi-Vendor, One Click MRI™ Automation Platform to Increase Real-Time MRI Imaging Access and Accuracy for Patients – New AHA and ACC Guidelines, Making Cardiac MRI a Class I Recommendation for Chest Pain, Will Increase CMR Use to Widely Impact the Practice of Cardiology PALO ALTO, Calif.–(BUSINESS WIRE)–HeartVista, […]
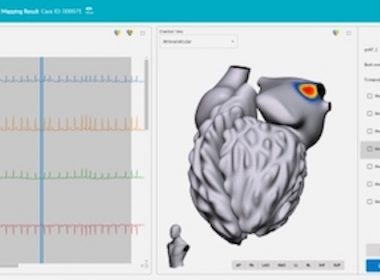
Vektor Medical Receives FDA Clearance for vMap™, First Technology Designed to Identify Arrhythmia Hot Spots Anywhere in Heart in Minutes Using Only ECG Data
Non-Invasive Technology Requires No Vests, CT or MRI Imaging, or Invasive Mapping SAN DIEGO–(BUSINESS WIRE)–Vektor Medical, Inc. today announced U.S. Food and Drug Administration (FDA) 510(k) clearance for its novel computational ECG mapping system, vMap™. vMap is designed to map potential arrhythmia sources (hot spots) associated with stable or unstable […]
DiaMedica Therapeutics Announces Addition of Stroke Recurrence as a Second Independent Primary Endpoint in ReMEDy2 Phase 2/3 AIS Trial
MINNEAPOLIS–(BUSINESS WIRE)–DiaMedica Therapeutics Inc. (Nasdaq: DMAC), a clinical-stage biopharmaceutical company focused on developing novel treatments for neurological disorders and kidney diseases, announced today that the U.S. Food and Drug Administration (FDA) has accepted and concluded that DiaMedica “may proceed” with the proposed clinical investigation using its amended protocol adding stroke […]