ANDOVER, Mass., Sept. 7, 2021 /PRNewswire/ — TransMedics Group, Inc. (“TransMedics”) (Nasdaq: TMDX), a medical technology company that is transforming organ transplant therapy for patients with end-stage lung, heart, and liver failure, today announced that the U.S. Food and Drug Administration (FDA) has granted premarket approval (PMA) of its OCS Heart System for […]
Regulatory
FDA Approves First-of-Its-Kind Stroke Rehabilitation System
SILVER SPRING, Md., Aug. 27, 2021 /PRNewswire/ — The U.S. Food and Drug Administration today approved the MicroTransponder Vivistim Paired VNS System (Vivistim System), a first-of-its-kind, drug-free rehabilitation system intended to treat moderate to severe upper extremity motor deficits associated with chronic ischemic stroke—a stroke caused by a blockage of blood flow […]
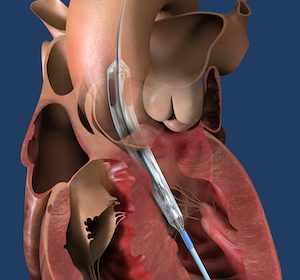
Medtronic Announces FDA Approval of Next-Gen TAVR System for Treatment of Symptomatic Severe Aortic Stenosis
Evolut™ FX TAVR System Adds Innovative Features to Enhance Ease-of-Use and Predictable Valve Deployment DUBLIN, Aug. 24, 2021 /PRNewswire/ — Medtronic plc (NYSE: MDT), the global leader in medical technology, today announced U.S. Food and Drug Administration (FDA) approval of its newest-generation, self-expanding transcatheter aortic valve replacement (TAVR) system, the Evolut™ FX TAVR system. […]
MFDS Greenlights VUNO Med®-DeepCARS™, AI Medical Device for Cardiac Arrest Prediction
SEOUL, South Korea, Aug. 24, 2021 /PRNewswire/ — South Korean artificial intelligence (AI) developer, VUNO Inc. announced today that it has received regulatory approval from the Ministry of Food and Drug Safety (MFDS) for its VUNO Med®–DeepCARS™, an AI medical device for cardiac arrest prediction through vital signs. VUNO Med®–DeepCARS™ is a […]
FDA Approves Expanded Peripheral Artery Disease (PAD) Indication for XARELTO® (rivaroxaban) Plus Aspirin to Include Patients After Lower-Extremity Revascularization (LER) Due to Symptomatic PAD
XARELTO® is the first and only therapy indicated for both coronary artery disease (CAD) and PAD, now including PAD patients post-LER XARELTO® is the only anticoagulant in 20 years to show significant benefit in patients with PAD who remain at high risk for major thrombotic events, including acute limb ischemia […]
FDA Completes On-site Pre-Approval Inspection of Liquidia’s Morrisville, North Carolina Facility
No Form 483 observations were issued during 5-day inspection MORRISVILLE, N.C., Aug. 18, 2021 (GLOBE NEWSWIRE) — Liquidia Corporation (NASDAQ: LQDA) announced today that the U.S. Food and Drug Administration (FDA) has completed an on-site Pre-Approval Inspection (PAI) of its Morrisville, North Carolina facility in connection with the on-going review […]
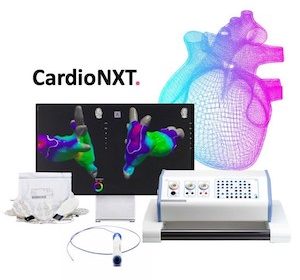
CardioNXT wins FDA nod for heart treatment delivery platform
BOULDER, Colo., Aug. 18, 2021 /PRNewswire/ — CardioNXT announced today that it received marketing clearance from the U.S. Food and Drug Administration (FDA) for the platform technology comprised of the iMap 3D Navigation & Mapping System™, Activate Software™, Sensor Enabled Axis Patient Patches™, and MultiLink Sensor Enabled Catheter™. The CardioNXT technology platform enables […]
FDA Grants Breakthrough Device Designation to Impella ECP, the World’s Smallest Heart Pump
DANVERS, Mass.–(BUSINESS WIRE)–The United States Food and Drug Administration (FDA) has granted breakthrough device designation to Abiomed’s (NASDAQ: ABMD) Impella ECP expandable percutaneous heart pump. The designation means the FDA will prioritize Impella ECP’s regulatory review processes including design iterations, clinical study protocols and pre-market approval (PMA) application. Impella ECP is the smallest […]
EchoNous Receives FDA Clearance for Lexsa Linear Probe, Designed for Kosmos Ultraportable Ultrasound Platform
Latest Addition to the Kosmos Platform Now Enables Full-Body Imaging at Point-of-Care in a Hand-Carried Device REDMOND, Wash.–(BUSINESS WIRE)–EchoNous, the leader in portable AI-guided ultrasound tools and software, announced today that the U. S. Food and Drug Administration (FDA) has cleared Lexsa, the company’s new 128-channel linear probe, for use […]
Helius Medical Technologies, Inc. Announces FDA Breakthrough Device Designation for the Treatment of Dynamic Gait and Balance Deficits Following a Stroke
NEWTOWN, Pa., Aug. 17, 2021 (GLOBE NEWSWIRE) — Helius Medical Technologies, Inc. (Nasdaq:HSDT) (TSX:HSM) (“Helius” or the “Company”), a neurotech company focused on neurological wellness, today announced that it has received Breakthrough Designation from the U.S. Food and Drug Administration (“FDA”) for its PoNS™ device with the proposed indication for […]