Clinical trial to pave the way for Synchron’s StentrodeTM to become first commercially available implantable brain computer interface NEW YORK–(BUSINESS WIRE)–Synchron, a venture-backed brain data transfer company, today announced that the U.S. Food and Drug Administration (FDA) has approved its Investigational Device Exemption (IDE) application for its flagship product, the StentrodeTM motor […]
Regulatory
ZOLL Medical Receives FDA Clearance for TBI Dashboard on Its Propaq M Monitor and Propaq MD Monitor/Defibrillator
CHELMSFORD, Mass.–(BUSINESS WIRE)–ZOLL® Medical Corporation, an Asahi Kasei company that manufactures medical devices and related software solutions, today announced that it has received FDA 510(k) clearance to release the TBI Dashboard™ feature on its Propaq® M monitor and Propaq MD monitor/defibrillator. TBI Dashboard provides clinical decision support for managing patients […]
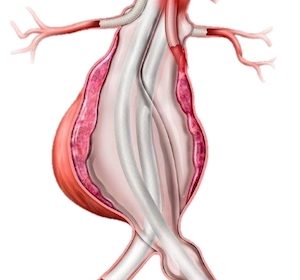
Endologix LLC Receives FDA Breakthrough Device Designation for ChEVAS™ System
Investigational EVAS System Designed for Patients with Complex Abdominal Aortic Aneurysm IRVINE, Calif.–(BUSINESS WIRE)–Endologix LLC, a leader in the treatment of vascular disease, today announced the company’s ChEVAS™ (Chimney EndoVascular Aneurysm Sealing) System has been granted a Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA). The ChEVAS […]
iRhythm® Technologies Comments on the Centers for Medicare and Medicaid Services 2022 Proposed Medicare Physician Fee Schedule
SAN FRANCISCO, July 14, 2021 (GLOBE NEWSWIRE) — iRhythm Technologies, Inc. (NASDAQ: IRTC), a leading digital healthcare solutions company focused on the advancement of cardiac care, today provided comment on the recently released Centers for Medicare and Medicaid Services (“CMS”) Calendar Year 2022 Medicare Physician Fee Schedule Proposed Rule (the […]
East End Medical Announces FDA Clearance Of Its SafeCross™ Transseptal RF Puncture And Steerable Balloon Introducer System
Novel 3-in-1 system provides predictable left atrium access to support transseptal interventions MIAMI, July 13, 2021 /PRNewswire/ — East End Medical, a private medical device company committed to improving catheter-based cardiac procedures, today announced it received U.S. Food and Drug Administration (FDA) clearance for the company’s SafeCross™ Transseptal Radiofrequency (RF) Puncture & Steerable Balloon […]
FDA Clears Personal ECG Device for Measurement of QTc Interval, a Critical Marker for Patient Safety
First of its Kind FDA Action Allows In-Office and At-Home Measurement for Potentially Life-Threatening Side Effect of Certain Medications MOUNTAIN VIEW, Calif., July 8, 2021 /PRNewswire/ — AliveCor, the global leader in FDA-cleared personal electrocardiogram (ECG) technology and services, today announced it received 510(k) clearance by the U.S. Food and Drug Administration (FDA) […]
CytoSorbents Receives Full FDA Approval of Investigational Device Exemption (IDE) for U.S. STAR-T Trial on Ticagrelor Removal During Cardiothoracic Surgery
MONMOUTH JUNCTION, N.J., July 6, 2021 /PRNewswire/ — CytoSorbents Corporation (NASDAQ: CTSO) announces the full approval of its Investigational Device Exemption (IDE) application to conduct the pivotal STAR-T (Safe and Timely Antithrombotic Removal – Ticagrelor) double-blind, randomized, controlled trial (RCT) in the United States to support FDA regulatory clearance. This study is being performed under the previously announced FDA Breakthrough […]
ABBOTT’S XIENCE STENT RECEIVES FDA APPROVAL FOR SHORTEST BLOOD THINNER COURSE FOR HIGH BLEEDING RISK PATIENTS
– New labeling approval for Abbott’s XIENCE™ stent builds on a legacy of safety and allows for one-month (as short as 28 days) dual anti-platelet therapy (DAPT) for patients at high bleeding risk (HBR) – The new XIENCE Skypoint™ stent received FDA approval in the U.S. and CE Mark approval […]
FDA Grants Highest Level of Approval to the Next Generation of Impella RP to Treat Right Heart Failure
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed’s (NASDAQ: ABMD) newest right heart pump, the Impella RP with SmartAssist, has received U.S. Food and Drug Administration (FDA) pre-market approval (PMA) as safe and effective to treat acute right heart failure for up to 14 days. Impella RP with SmartAssist is the first single-access temporary percutaneous ventricular […]
FDA Clears the Smallest Life-Saving Bleeding Control Device, COBRA-OS™, by Front Line Medical Technologies
After three years of research and development, Front Line Medical Technologies introduces the world’s smallest REBOA device to control bleeding and help patients survive emergencies LONDON, Ontario, June 29, 2021 (GLOBE NEWSWIRE) — Front Line Medical Technologies, Inc. announced today that the U.S. Food and Drug Administration (FDA) has cleared its COBRA-OS™ […]