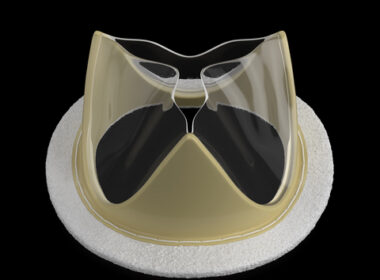
SALT LAKE CITY–(BUSINESS WIRE)–Foldax® Inc., a leader in heart valve innovation, today announced that the Indian Central Drugs Standard Control Organization (CDSCO) approved its TRIA™ Mitral Valve. Dolphin Life Science India LLP will locally manufacture the TRIA Mitral Valve in India. Foldax’s vision for its novel polymer heart valves is […]
Regulatory
HeartSciences Receives FDA Breakthrough Device Designation for MyoVista Insights AI-ECG Algorithm for Detecting Aortic Stenosis
Aortic Stenosis is a Serious and Widespread Condition; The AI-ECG Algorithm Offers a Powerful Diagnostic Solution Designed for Seamless Integration with Hospital EHR Systems Aortic Stenosis is a Serious and Widespread Condition; The AI-ECG Algorithm Offers a Powerful Diagnostic Solution Designed for Seamless Integration with Hospital EHR Systems
FDA Grants De Novo Clearance for Reflow Medical’s Spur® Peripheral Retrievable Stent System
San Clemente, CA – May 29, 2025 – Reflow Medical, Inc., a leading developer of innovative medical devices focused on complex cardiovascular disease, announced that the U.S. Food and Drug Administration (FDA) has granted De Novo clearance for the company’s Spur Peripheral Retrievable Stent System a unique clinical solution for the treatment of […]
ZYLOX Unicorn™ Vascular Closure Device Receives Regulatory Approval in Indonesia
HANGZHOU, China, May 26, 2025 /PRNewswire/ — Zylox-Tonbridge Medical Technology Co., Ltd. (“Zylox-Tonbridge” or the “Company”) today announced that its proprietary ZYLOX Unicorn™ Vascular Closure Device (VCD) has received regulatory approval in Indonesia. This marks the ZYLOX Unicorn™…
CytoSorbents Provides Regulatory Update for DrugSorb-ATR
PRINCETON, N.J., May 1, 2025 /PRNewswire/ — CytoSorbents Corporation (NASDAQ: CTSO), a leader in blood purification therapies for life-threatening conditions in the intensive care unit and cardiac surgery, today provided a regulatory update for DrugSorb™-ATR, its FDA-designated…
Vivasure Medical Receives CE Mark Approval in Europe for PerQseal Elite Vascular Closure System
First fully absorbable, sutureless closure system designed for large-bore procedures aims to improve procedural efficiency and patient outcomes in structural heart interventions GALWAY, Ireland–(BUSINESS WIRE)–Vivasure Medical®, a company pioneering novel fully absorbable technology for percutaneous vessel closure, today announced European CE mark approval of the PerQseal® Elite vascular closure system, a […]
CardioVia Announces FDA Clearance for ViaOne, Opening a New Frontier in Minimally Invasive Heart-Surface Treatments
TEL AVIV, Israel, April 8, 2025 /PRNewswire/ — CardioVia, an innovative medical device company specializing in advanced cardiac care solutions, announced today that it has received U.S. Food and Drug Administration (FDA) clearance for its ViaOne system. This breakthrough device is…
Brainomix Advances Stroke Care with Landmark FDA Clearance
Brainomix 360 unlocks expert-level insights from universally available non-contrast CT scans, helping stroke networks expand access to life-changing treatments OXFORD, England and CHICAGO, April 8, 2025 /PRNewswire/ — Brainomix, a global leader in AI-powered stroke imaging, has announced…
Q’Apel Medical Announces CE Mark Approval for Armadillo SelectFlex™ Neurovascular Access System
Patented SelectFlex™ Technology allows operators to achieve the ideal balance of trackability and support on demand to navigate complex neurovasculature System enables biaxial approach, regardless of access site, to eliminate complexity and cost of conventional configurations FREMONT,…
Corvia Medical Achieves CE Certification for Atrial Shunt Under New EU MDR Standards
Commercial validation in Europe parallels ongoing RESPONDER-HF clinical trial TEWKSBURY, Mass., Dec. 20, 2024 /PRNewswire/ — Corvia Medical, Inc., a company dedicated to transforming the treatment of heart failure, announced today that it has achieved CE certification for the Corvia®…