TARRYTOWN, N.Y., Feb. 11, 2021 /PRNewswire/ — Homozygous familial hypercholesterolemia (HoFH) is an ultra-rare inherited condition that affects approximately 1,300 patients in the U.S. and is characterized by extremely high low-density lipoprotein cholesterol (LDL-C) In pivotal Phase 3 HoFH trial, adding Evkeeza to standard lipid-lowering therapies reduced LDL-C by nearly half at 24 […]
Regulatory
CARMAT Receives FDA Approval to Use the New Version of Its Artificial Heart in the US Early Feasibility Study (EFS)
First enrollment in the study expected in Q1 2021 PARIS–(BUSINESS WIRE)–Regulatory News: CARMAT (FR0010907956, ALCAR) (Paris:ALCAR), the designer and developer of the world’s most advanced total artificial heart, aiming to fulfill an unmet medical need by providing a therapeutic alternative to people suffering from end-stage biventricular heart failure, today provides […]
Puzzle Medical Devices Inc. Receives U.S. Food and Drug Administration (FDA) Breakthrough Device Designation for Its Revolutionary Minimally Invasive Transcatheter Heart Pump
MONTREAL, Feb. 8, 2021 /PRNewswire/ – Puzzle Medical Devices Inc., (www.puzzlemed.com) announced today that the U.S. Food and Drug Administration (FDA) has granted the company a Breakthrough Device Designation for its revolutionary transcatheter pump to address heart failure. The FDA Breakthrough Device Program is intended to help patients receive more timely access […]
B-Secur Receives FDA Regulatory Clearance For Its HeartKey® EKG/ECG Technology
Expands portfolio across Health, Wellness and User Identification for deployment in consumer and medical technology sectors BELFAST, Northern Ireland–(BUSINESS WIRE)–B-Secur, a leader in EKG/ECG technology, today announced that it has received U.S. Food and Drug Administration (FDA) 510(K) clearance of its HeartKey® software library1. B-Secur’s HeartKey® is a suite of powerful […]
Medtronic Receives FDA Approval of DiamondTemp Ablation System for the Treatment of Patients with Atrial Fibrillation
Clinical Trial Demonstrates Procedural Efficiencies, Safety, Effectiveness and Non-Inferiority of DiamondTemp System DUBLIN, Jan. 29, 2021 /PRNewswire/ — Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced it has received U.S. Food and Drug Administration (FDA) approval of the DiamondTemp™ Ablation (DTA) system which treats patients with recurrent, symptomatic paroxysmal atrial […]
Alleviant Medical Receives Breakthrough Device Designation From FDA for Transcatheter Technology
Innovative technology offers an implant-free approach for individuals with chronic heart failure AUSTIN, Texas–(BUSINESS WIRE)–Alleviant Medical Inc., a privately-held medical device company, today announced that the US Food and Drug Administration (FDA) has granted the company a Breakthrough Device designation for its transcatheter technology. The technology offers a no-implant interatrial […]
PEDRA™ Technology Receives FDA Breakthrough Device Designation for its PEDRA™ Xauron™ Real-Time Tissue Perfusion System
Novel perfusion monitor achieves FDA Breakthrough Device Designation for real-time, periprocedural monitoring of tissue perfusion in patients with critical limb threatening ischemia SINGAPORE, Jan 25, 2021 /PRNewswire/ — PEDRA™ Technology, a privately-held company, announced today that the U.S Food and Drug Administration (FDA) has granted the company a Breakthrough Device Designation for the […]
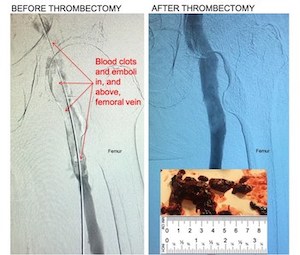
Kishor Vora, M.D. Owensboro Heart & Vascular, Removes Large Blood Clots in 1st Global Use of New Device
11F mechanical thrombectomy system removes large blood clots in deep vein thrombosis (DVT) HALLANDALE BEACH, Flo., Jan. 22, 2021 /PRNewswire/ — Control Medical Technology announced the FDA cleared Control 11F Mechanical Thrombectomy system was used to remove large blood clots from patients with deep vein thrombosis (DVT). “Control removed large blood […]
Canon Medical’s AI-Powered, Premium Large Bore CT Receives FDA Clearance
Aquilion Exceed LB CT System Offers Industry’s Largest Bore and Widest Field-of-View TUSTIN, Calif.–(BUSINESS WIRE)–Canon Medical Systems USA, Inc. has received FDA clearance for the Aquilion Exceed LB™ CT system, giving clinicians the opportunity to see more during radiation therapy planning for accuracy, precision and speed. As cancer cases continue to […]
Merck Announces U.S. FDA Approval of VERQUVO® (vericiguat)
VERQUVO Approved for Reduction of Risk of Cardiovascular Death and Heart Failure (HF) Hospitalization Following a Hospitalization for HF or Need for Outpatient Intravenous (IV) Diuretics in Adults with Symptomatic Chronic Heart Failure and Ejection Fraction Less than 45% VERQUVO is the First Soluble Guanylate Cyclase Stimulator, Approved to Treat […]