United Imaging now preferred medical imaging partner for MyoStrain® and its unique diagnostic cardiac test. HOUSTON, Aug. 5, 2020 /PRNewswire/ — United Imaging, a global leader in advanced medical imaging and radiotherapy equipment, along with Myocardial Solutions, a medical technology company working to transform the cardiac and cancer care continuum, announced today […]
Regulatory
FDA Issues Emergency Use Authorization for Impella Heart Pumps to Provide Unloading Therapy to COVID-19 Patients
DANVERS, Mass.–(BUSINESS WIRE)–The United States Food and Drug Administration (FDA) has issued an emergency use authorization (EUA) for left-sided Impella heart pumps to provide left ventricular unloading and support to COVID-19 patients who are undergoing ECMO treatment and develop pulmonary edema or myocarditis. Impella is manufactured by Abiomed (NASDAQ: ABMD). COVID-19 causes widespread inflammation which can […]
Biotricity Receives FDA clearance for Bioflux Software II System
The software significantly improves workflows for analysis, increasing efficiency and reducing costs REDWOOD CITY, Calif., Aug. 04, 2020 (GLOBE NEWSWIRE) — Biotricity Inc. (OTCQB: BTCY), a medical diagnostic and consumer healthcare technology company, today announced that it has received a 510(k) clearance from the FDA for its Bioflux Software II System. The software […]
Stryker’s Neuroform Atlas® Stent System granted an expanded indication, providing a new option for patients with aneurysms in the back of the brain
KALAMAZOO, Michigan, USA, Aug. 3, 2020 /PRNewswire/ — Stryker announced today that it has received U.S. Food and Drug Administration (FDA) approval for an expanded indication of its Neuroform Atlas Stent System, becoming the first and only adjunctive stent approved for use in the posterior (back of the brain) circulation. Aneurysms in the posterior […]
scPharmaceuticals Announces FDA Acceptance of FUROSCIX® New Drug Application Resubmission
FDA sets PDUFA date of December 30, 2020 BURLINGTON, Mass.–(BUSINESS WIRE)–scPharmaceuticals Inc. (Nasdaq: SCPH), a pharmaceutical company focused on developing and commercializing products that have the potential to optimize the delivery of infused therapies, advance patient care, and reduce healthcare costs, today announced that the U.S. Food and Drug Administration […]
MyoKardia Announces Receipt of Breakthrough Therapy Designation from FDA for Mavacamten for the Treatment of Symptomatic, Obstructive Hypertrophic Cardiomyopathy
BRISBANE, Calif., July 23, 2020 (GLOBE NEWSWIRE) — MyoKardia, Inc. (Nasdaq: MYOK) today announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Therapy Designation to mavacamten, a novel, oral, allosteric modulator of cardiac myosin, for the treatment of symptomatic, obstructive hypertrophic cardiomyopathy (HCM). The FDA’s Breakthrough Therapy […]
Caption Health Receives FDA 510(k) Clearance for Innovation in Point-of-Care Ejection Fraction Evaluation
Latest AI ultrasound tool makes it even easier to automatically assess ejection fraction, a key indicator of cardiac function, at the bedside–including on the front lines of the COVID-19 pandemic BRISBANE, Calif., July 23, 2020 /PRNewswire/ — Caption Health, a leader in medical AI technology, has received FDA 510(k) clearance for an […]
Boston Scientific Receives FDA Approval for Next-Generation WATCHMAN FLX™ Left Atrial Appendage Closure Device
New stroke risk reduction technology designed to advance procedural performance and safety, treat wider range of patients with non-valvular atrial fibrillation MARLBOROUGH, Mass., July 21, 2020 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) announced it has received U.S. Food and Drug Administration (FDA) approval for the WATCHMAN FLX™ Left Atrial Appendage Closure (LAAC) Device. Experience […]
Canon Medical’s 1.5T MR System Receives FDA Clearance for AI-Based Image Reconstruction Technology
Vantage Orian 1.5T the Latest System in Company’s Portfolio with Access to Advanced intelligent Clear-IQ Engine (AiCE) TUSTIN, Calif.–(BUSINESS WIRE)–Canon Medical Systems USA, Inc. has received 510(k) clearance on its Advanced intelligent Clear-IQ Engine (AiCE)* for the Vantage Orian 1.5T MR system, continuing to expand access to its new Deep Learning Reconstruction (DLR) […]
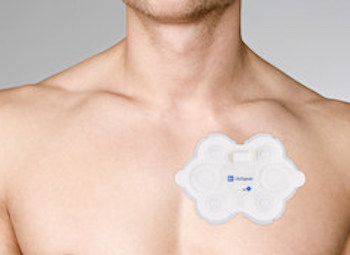
LifeSignals Wireless Medical Biosensor LP1250 receives FDA clearance
– World’s first single-use two channel ECG and heart rate biosensor provides 72-hour patient monitoring with remote data access in ambulatory, hospital and home settings FREMONT, Calif., July 21, 2020 /PRNewswire/ — LifeSignals Group, today announced USFDA 510(k) clearance has been received for their LifeSignals ECG Remote Monitoring Patch Platform. The LifeSignals […]