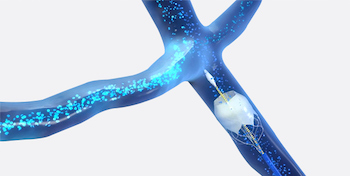
IRVINE, Calif., July 15, 2020 /PRNewswire/ — Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced it received approval from the U.S. Food and Drug Administration (FDA) for the KONECT RESILIA aortic valved conduit (AVC), the first ready-to-implant solution for bio-Bentall procedures, […]
Regulatory
FDA Grants the Doraya AHF Catheter Breakthrough Designation
NETANYA, Israel, July 14, 2020 /PRNewswire/ — Revamp Medical, a medical device company developing a percutaneous device for the treatment of acute heart failure (AHF), today announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for the Doraya™ device. The Doraya is a temporary catheter, placed in the […]
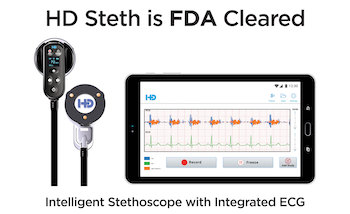
HD Medical Awarded FDA Clearance For HD Steth
Intelligent Stethoscope with Integrated ECG delivers Instant Cardiac Insights SANTA CLARA, Calif.–(BUSINESS WIRE)–HD Medical, Inc. of Silicon Valley today announces that its flagship product, HD Steth, has received FDA clearance for all three product classification codes of DQD, DQC and DPS for Electronic Stethoscope, Phonocardiograph and Electrocardiograph combined into one device. HD Steth utilizes […]
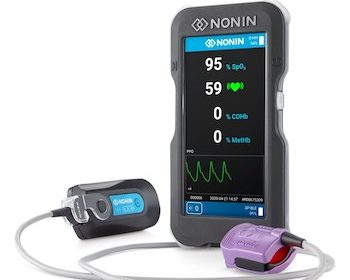
Nonin Medical Announces FDA Clearance of the CO-Pilot™ Wireless Handheld Multi-Parameter System
Device and sensors help EMS, fire departments and military track five patient parameters MINNEAPOLIS, July 8, 2020 /PRNewswire/ — Nonin Medical, Inc. today announced the CO-Pilot™ Wireless Handheld Multi-Parameter System (H500) received 510(k) clearance from the U.S. Food and Drug Administration (FDA). The CO-Pilot is a handheld device with wireless capabilities that helps […]
Medtronic Gains FDA Clearance, CE Mark for LINQ II™ Insertable Cardiac Monitor (ICM)
Next Generation ICM Offers Remote Programming with Improved Longevity and Enhanced Accuracy DUBLIN, July 07, 2020 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced it has received U.S. Food and Drug Administration (FDA) clearance and CE (Conformité Européenne) Mark approval for its LINQ II™ insertable cardiac monitor (ICM) with […]
Abbott Receives FDA Approval for New Heart Rhythm Devices Featuring Bluetooth Connectivity and Continuous Remote Monitoring
ABBOTT PARK, Ill., July 6, 2020 /PRNewswire/ — Abbott (NYSE: ABT) today announced that the U.S. Food and Drug Administration (FDA) has approved the company’s next-generation Gallant™ implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy defibrillator (CRT-D) devices. The devices bring new benefits to patients with heart rhythm disorders, including […]
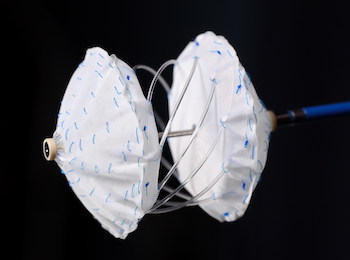
Carag Receives U.S. FDA IDE Approval to Conduct Clinical Study of First Transcatheter Septal Occluder With Bioresorbable, Metal-free Framework
The U.S. trial for CE-marked CBSO is designed to enroll up to 250 patients in a staged study approach BAAR, Switzerland, July 1, 2020 /PRNewswire/ — CARAG AG, a privately-held Swiss medical device development company, today announced receiving U.S. Food and Drug Administration (FDA) Investigational Device Exemption (IDE) approval for its Carag Bioresorbable […]
MedAlliance Gains CE Mark Approval for Coronary SELUTION SLR™ Sirolimus Drug Eluting Balloon
NYON, Switzerland, June 30, 2020 /PRNewswire/ — MedAlliance has announced the award of its second CE Mark: SELUTION SLR™ 014 PTCA, a novel Sirolimus Drug Eluting Balloon (DEB), for the treatment of coronary arterial disease. This includes indications for both de-novo lesions as well as in-stent restenosis. The approval applies to a broad […]
Boston Scientific Receives FDA 510(k) Clearance for the LUX-Dx™ Insertable Cardiac Monitor System
First ICM device with remote programming paired with dual-stage arrhythmia detection algorithm MARLBOROUGH, Mass., June 29, 2020 /PRNewswire/ — Boston Scientific (NYSE: BSX) has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the LUX-Dx™ Insertable Cardiac Monitor (ICM) System, a new, long-term diagnostic device implanted in patients to detect arrhythmias associated with […]
preCARDIA, Inc. Receives FDA Breakthrough Device Designation for Novel, Catheter Based Heart Failure Treatment
Pioneering technology is designed to address Acute Decompensated Heart Failure ST. PAUL, Minn., June 23, 2020/PRNewswire/ — preCARDIA, Inc., has announced that the company’s catheter based system for treating volume overload in patients with acutely decompensated heart failure (ADHF) has been designated for the Breakthrough Devices Program by the U.S. […]