Amsterdam, the Netherlands and Cambridge, MA – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced that the Center for Devices and Radiological Health (CDRH) of the U.S. Food and Drug Administration (FDA) has granted premarket approval (PMA) for the company’s HeartStart FR3 [1] and HeartStart FRx [2] […]
Regulatory
Resverlogix Reaches Agreement With FDA for Key Aspects of Apabetalone Registration Enabling Study
CALGARY, Alberta, June 22, 2020 (GLOBE NEWSWIRE) — Resverlogix Corp. (“Resverlogix”) (TSX:RVX) is pleased to announce that the U.S. Food & Drug Administration (FDA) has accepted its BETonMACE2 clinical plan as a registration enabling study with positive implications for Resverlogix and its ongoing partnership discussions. Key written development points from […]
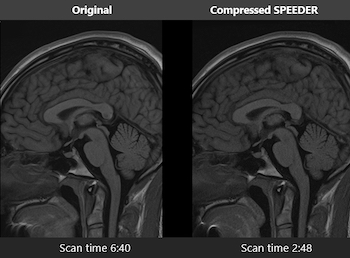
Canon Medical Receives FDA Clearance for Compressed SPEEDER Technology for 1.5T MR, Enabling Reduced Scan Times
New Software Version for Vantage Orian 1.5T Also Offers Cybersecurity Solutions to Protect Patient Data TUSTIN, Calif.–(BUSINESS WIRE)–Hospitals and institutions are continually looking for ways to improve diagnostic imaging throughput, especially in today’s environment where disinfection of systems and rooms in between patients is crucial. Now, with the newly FDA-cleared […]
MIVI Neuroscience Announces FDA IDE Clinical Study Approval For the MIVI Q Aspiration Catheter
EDEN PRAIRIE, Minn., June 9, 2020 /PRNewswire/ — MIVI Neuroscience, a leading developer, and marketer of precision stroke therapy devices utilized by neurointerventionists around the world, announced today that the FDA has determined the company has provided sufficient data to support initiation of a human clinical study of the MIVI Q Aspiration Catheter […]
FDA Approves Abiomed’s First-in-Human Trial of Impella ECP, World’s Smallest Heart Pump
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ: ABMD) announces the United States Food and Drug Administration (FDA) has approved the company’s investigational device exemption application to start an early feasibility study with a first-in-human trial of the 9 French (Fr) Impella ECP™ heart pump. Impella ECP, which stands for expandable cardiac power, will be […]
Novadoz Pharmaceuticals FDA Approvals of Oseltamivir and Dofetilide Continues the Company’s Rise in the Generic Market
PISCATAWAY, N.J., June 4, 2020 /PRNewswire/ — Novadoz Pharmaceuticals, the U.S based sales & marketing affiliate for MSN Labs, located in Hyderabad India, recently received FDA approval for their generic versions of Oseltamivir Phosphate Capsules and Dofetilide Capsules. The company was notified earlier this year, that Dofetilide 125mcg, 250mcg, 500mcg capsules, AB rated to […]
FDA Issues Emergency Use Authorization for Impella RP as Therapy for COVID-19 Patients with Right Heart Failure
DANVERS, Mass.–(BUSINESS WIRE)–The United States Food and Drug Administration (FDA) has issued an emergency use authorization (EUA) for Impella RP to include patients suffering from COVID-19 related right heart failure or decompensation, including pulmonary embolism (PE). Abiomed (NASDAQ: ABMD) manufactures Impella RP. Impella RP is a temporary heart pump that provides circulatory support for […]
Thermedical’s Groundbreaking SERF Ablation System Earns FDA’s Breakthrough Device Designation
WALTHAM, Mass.–(BUSINESS WIRE)–Thermedical®, a developer of thermal-ablation medical systems to treat ventricular tachycardia (VT), today announced that it has received Breakthrough Device Designation from the U.S. Food & Drug Administration (FDA) for its Saline Enhanced Radiofrequency (SERF) Ablation system and Durablate® catheter. The FDA Breakthrough Devices Program is intended to help patients […]
Philips receives FDA clearance for the use of its ultrasound portfolio to manage COVID-19-related lung and cardiac complications
May 13, 2020 Philips’ ultrasound portfolio, including Lumify with Reacts handheld tele-ultrasound solution, provides valuable diagnostic insight for front-line care providers Amsterdam, the Netherlands – Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration […]
FDA Clears New XO Cross Microcatheter Platform
New non-tapered metal-alloy catheter platform FDA cleared for use in peripheral vasculature PARK CITY, Utah, May 12, 2020 /PRNewswire/ — Transit Scientific announced the FDA cleared the XO Cross® Microcatheter platform for guidewire support, exchange, and contrast media injection in the peripheral vasculature. Microcatheters are small 0.70-1.30mm outer diameter (OD) catheters used to provide guidewire […]