– The CATALYST trial will examine Abbott’s Amplatzer™ Amulet™ device compared to non-vitamin K oral anticoagulants, the current standard in attempting to lower stroke and bleeding risks for patients with atrial fibrillation ABBOTT PARK, Ill., Feb. 3, 2020 /PRNewswire/ — Abbott (NYSE: ABT) today announced that the U.S. Food and Drug Administration […]
Regulatory
Ancora Heart Announces Expansion of U.S. Early Feasibility Study
CorCinch HFrEF Study Evaluating Safety of AccuCinch Ventricular Repair System in Heart Failure Patients SANTA CLARA, Calif.–(BUSINESS WIRE)–Ancora Heart, Inc., a company developing a novel therapy to address heart failure, today announced that the U.S. Food and Drug Administration (FDA) has approved an expansion of enrollment in its CorCinch HFrEF […]
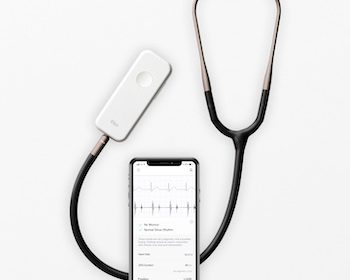
FDA Clears Eko’s AFib and Heart Murmur Detection Algorithms, Making It the First AI-Powered Stethoscope to Screen for Serious Heart Conditions
Eko’s algorithms alert clinicians to the presence of heart murmurs and atrial fibrillation (AFib) during the physical exam, converting the classic stethoscope into a powerful early detection tool SAN FRANCISCO–(BUSINESS WIRE)–Eko, a digital health company applying artificial intelligence (AI) in the fight against heart disease, announced today that the U.S. […]
Intact Vascular Expands Tack Endovascular System® Portfolio Offering
New Larger Implant Enables Focal Dissection Repair Across a Wider Range of Vessel Sizes WAYNE, Pa.–(BUSINESS WIRE)–Intact Vascular, Inc., a developer of medical devices for minimally invasive peripheral vascular procedures, today announced Food and Drug Administration PMA approval for the expansion of its Tack Endovascular System® (6F) portfolio. The new approved device […]
Verily receives FDA 510(k) clearance for Study Watch with Irregular Pulse Monitor
One year ago, we received our first 510(k) clearance for ECG for Verily Study Watch — a wrist-worn, sensor-based device for non-invasive, continuous monitoring. Today, we’re thrilled to share that we’ve received a new 510(k) clearance for Study Watch with Irregular Pulse Monitor. This validates our approach at Verily to building robust, clinical […]
Medtronic Receives FDA Approval for Trial Evaluating New Energy Source with Pulsed Electric Fields to Treat Atrial Fibrillation
Investigative Technology Designed to Interrupt Irregular Pathways in the Heart DUBLIN, Jan. 23, 2020 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) today announced that it received approval from the U.S. Food and Drug Administration (FDA) to proceed with an investigational device exemption (IDE) trial to evaluate the safety and effectiveness of the PulseSelect™ Pulsed Field […]
Teleflex Receives FDA Clearance for Wattson™ Temporary Pacing Guidewire
WAYNE, Pa., Jan. 22, 2020 (GLOBE NEWSWIRE) — Teleflex Incorporated (NYSE: TFX), a leading global provider of medical technologies for critical care and surgery, today announced that it received 510(k) clearance from the U.S. Food and Drug Administration for the WattsonTM Temporary Pacing Guidewire – the first commercially available bipolar temporary […]
FDA Approves Medtronic Micra™ AV, the World’s Smallest Pacemaker Which Can Now Treat AV Block
With FDA Approval, More Patients in the U.S. Are Now Candidates for a Leadless Pacing Option DUBLIN, Jan. 21, 2020 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) today announced it has received U.S. Food and Drug Administration (FDA) approval of Micra™ AV, the world’s smallest pacemaker with atrioventricular (AV) synchrony. Micra AV is […]
Biotricity Prepares its Filing for a 510(k) Application for its Advanced ECG Analysis Software
REDWOOD CITY, Calif., Jan. 22, 2020 (GLOBE NEWSWIRE) — Biotricity Inc. (OTCQB: BTCY), a medical diagnostic and consumer healthcare technology company, announced today that it is finalizing its FDA filing for its next generation, advanced ECG analysis software with an expectation to file a 510(k) clearance application with the US FDA by end […]
European Commission Grants BioVentrix CE Mark Extension for Revivent TC Less Invasive Ventricular Enhancement Therapy
SAN RAMON, Calif.–(BUSINESS WIRE)–BioVentrix, Inc., developer of the first transcatheter device for left ventricular remodeling after a heart attack, today announced the extension of its CE Mark for the Revivent TC™ Transcatheter Ventricular Enhancement System for heart failure to May 2024. “The extension of our CE Mark is not only an important […]