NASDAQ: CORV TSX: CORV VANCOUVER, Dec. 10, 2019 /PRNewswire/ – Correvio Pharma Corp. (NASDAQ: CORV) (TSX: CORV), a specialty pharmaceutical company focused on commercializing hospital drugs, today announced that NASDAQ and the Toronto Stock Exchange (TSX) have halted trading of the Company’s common stock. The U.S. Food and Drug Administration (FDA)’s Cardiovascular and Renal […]
Regulatory
Bracco Diagnostics Inc.’s LUMASON® (sulfur hexafluoride lipid-type A microspheres) for injectable suspension, for intravenous use or intravesical use, receives U.S. Food and Drug Administration approval for use in echocardiography to opacify the left ventricular chamber and to improve the delineation of the left ventricular endocardial border in pediatric patients with suboptimal echocardiograms
MONROE TOWNSHIP, N.J., Dec. 2, 2019 /PRNewswire/ — Bracco Diagnostics Inc., the U.S. subsidiary of Bracco Imaging S.p.A., one of the world’s leading companies in the diagnostic imaging business, announced today that its contrast agent LUMASON is the first ultrasound enhancing agent (UEA) to obtain U.S. Food and Drug Administration (FDA) approval for […]
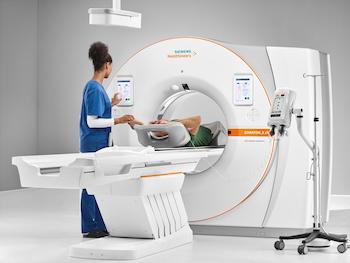
FDA Clears Siemens Healthineers SOMATOM X.cite CT Scanner With Intelligent User Interface Concept
myExam Companion intelligent user interface concept guides technologists through complex clinical tasks myExam Companion automatically adapts scans based on A.I.-trained clinical decision trees to simplify image acquisition parameters and reconstruction tasks SOMATOM X.cite improves patient comfort with an 82cm gantry bore and delivers powerful performance with Vectron tube MALVERN, Pa.–(BUSINESS […]
Medtronic Drug-Coated Balloon Receives U.S. FDA Approval to Treat Arteriovenous Fistula Lesions
Clinical Data Demonstrates IN.PACT™ AV DCB Is Safe, Reduces Reinterventions, and Maintains Access for End-Stage Renal Disease Patients Undergoing Dialysis DUBLIN, Nov. 21, 2019 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) today announced U.S. Food and Drug Administration (FDA) approval of the IN.PACT™ AV drug-coated balloon (DCB), a paclitaxel-coated balloon indicated for the […]
Merit Medical Receives FDA Breakthrough Device Designation for WRAPSODY™ Endovascular Stent Graft System
SOUTH JORDAN, Utah, Nov. 21, 2019 (GLOBE NEWSWIRE) — Merit Medical Systems, Inc. (NASDAQ: MMSI), a leading manufacturer and marketer of proprietary disposable devices used in interventional, diagnostic and therapeutic procedures, particularly in cardiology, radiology, oncology, critical care and endoscopy, announced today that it has been granted Breakthrough Device Designation […]
FDA Grants Breakthrough Device Designation Status for BioVentrix Revivent TC Transcatheter Ventricular Enhancement System for Heart Failure
SAN RAMON, Calif.–(BUSINESS WIRE)–BioVentrix, Inc., a privately-held company with a first-in-class, transcatheter-based structural heart device to treat heart failure, today announced that the U.S. Food and Drug Administration (FDA) has granted the company Breakthrough Device Designation status for its Revivent TC™ Transcatheter Ventricular Enhancement System for heart failure. Less Invasive […]
Amarin Announced FDA Advisory Committee Voted Unanimously (16-0) to Recommend Approval of Vascepa® (icosapent ethyl) Capsules Label Expansion to Reduce Cardiovascular Risk Based on Landmark REDUCE-IT® Outcomes Trial
Cardiovascular disease events like heart attacks, stroke and death affect millions of patients in the United States and are estimated to cost $500 billion annually Millions of high-risk patients with cardiovascular disease could benefit from this cost-effective therapy if expanded label receives FDA approval; PDUFA date is December 28 DUBLIN, […]
Ultromics Receives FDA Clearance for its AI-powered Decision Support System, EchoGo Core
AI automates cardiac analysis helping earlier detection of cardiovascular disease, enabling clinicians to improve patient care and outcomes OXFORD, England, Nov. 14, 2019 /PRNewswire/ — Ultromics, the UK-based health technology firm at the forefront of applying artificial intelligence to echocardiography, has received 510(K) clearance from the U.S. Food and Drug Administration (FDA) for […]
Okami Medical Announces Major Milestones: FDA 510(k) Clearance And Key Patent For The LOBO Vascular Occluder
Microcatheter-delivered device designed to provide rapid and focal occlusion of a wide range of peripheral arterial targets ALISO VIEJO, Calif., Nov. 4, 2019 /PRNewswire/ — Okami Medical Inc., a medical device company, today announced 510(k) clearance by the U.S. Food and Drug Administration (FDA) and initial launch of the LOBOTM Vascular Occlusion System. […]
GE Healthcare Announces U.S. FDA Approval of Macrocyclic MRI Contrast Agent Clariscan™ (gadoterate meglumine) Injection for Intravenous Use
Clariscan, approved in more than 55 countries globally with over four million patient doses shipped, is now FDA-approved in the U.S. Expands the GE Healthcare portfolio of contrast media products CHALFONT ST GILES, England–(BUSINESS WIRE)–The U.S. Food and Drug Administration (FDA) has approved Clariscan™, a macrocyclic, ionic, gadolinium-based, MRI contrast agent, […]