VANCOUVER, Sept. 12, 2019 /PRNewswire/ – Correvio Pharma Corp. (NASDAQ: CORV) (TSX: CORV), a specialty pharmaceutical company focused on commercializing hospital drugs, today highlighted that the U.S. Food and Drug Administration (FDA) has accepted for review United Therapeutics Corporation’s (NASDAQ: UTHR) New Drug Application (NDA) for Trevyent® (treprostinil) for the treatment of pulmonary arterial hypertension […]
Regulatory
V-Wave Receives Second FDA Breakthrough Device Designation: Interatrial Shunt for Pulmonary Arterial Hypertension
CAESAREA, Israel, Sept. 12, 2019 /PRNewswire/ — V-Wave Ltd., a privately held medical device company developing novel implantable interatrial shunt devices, today announced that the U.S. Food and Drug Administration (FDA) has granted the company a second Breakthrough Device Designation – the first was for its interatrial shunt for Heart Failure (HF), and […]
FDA Grants EBR Systems Breakthrough Device Designation Status for the WiSE Cardiac Resynchronization Therapy (CRT) System
SUNNYVALE, Calif.–(BUSINESS WIRE)–EBR Systems, Inc., developer of the world’s only wireless cardiac pacing system for heart failure, today announced that the U.S. Food and Drug Administration (FDA) has granted BreakthrouThe FDA created this designation and its associated program in 2017 for certain devices providing more effective treatment of life-threatening or […]
SoniVie Receives FDA Breakthrough Device Designation for the TIVUS System in the Treatment of Pulmonary Arterial Hypertension (PAH)
– Designation facilitates more rapid approval and reimbursement for a breakthrough technology that offers significant advantages over current PAH treatment options – TEL AVIV, ISRAEL (PRWEB) SEPTEMBER 06, 2019 SoniVie, an Israeli company developing a novel system for the treatment of PAH, today announced that it has been granted Breakthrough Device […]
Netech Obtains FDA 510(k) Clearance for Delta 3300 – Defibrillator/Pacemaker Analyzer
FARMINGDALE, N.Y., Sept. 9, 2019 /PRNewswire/ — Leading biomedical test-instrument manufacturer Netech announced today the launch of its new state-of-the-art defibrillator/pacemaker analyzer—the Delta 3300. Recently approved by the FDA 510(k), the Delta 3300 is a precision instrument for testing and validating the functions of all semi and automated defibrillators. This revolutionary compact, […]
Acceleron Receives FDA Orphan Drug Designation for Sotatercept in Pulmonary Arterial Hypertension
CAMBRIDGE, Mass.–(BUSINESS WIRE)–Acceleron Pharma Inc. (NASDAQ:XLRN), a leading biopharmaceutical company in the discovery and development of TGF-beta superfamily therapeutics to treat serious and rare diseases, today announced that the United States Food and Drug Administration (FDA) has granted Orphan Drug designation to sotatercept for the treatment of patients with pulmonary […]
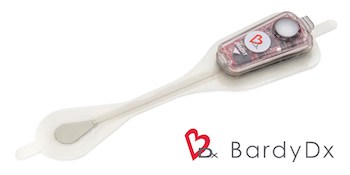
Bardy Diagnostics™ Receives FDA 510(k) Clearance for 14-Day Carnation Ambulatory Monitor (CAM) Patch
SEATTLE, Sept. 6, 2019 /PRNewswire/ — Bardy Diagnostics, Inc., (“BardyDx”), a leading provider of ambulatory cardiac monitoring technologies and custom data solutions, announced today it received 510(k) clearance from the U.S. Food and Drug Administration (“FDA”) for the 14-Day version of the Carnation Ambulatory Monitor (“CAM™”), the industry’s only P-wave centric™ ambulatory cardiac patch monitor and arrhythmia […]
NobleStitch™ EL Receives Approval Of IDE To Conduct A Trial For Reduction Of Recurrent Ischemic Stroke
NOBLES MEDICAL TECHNOLOGIES II, INC. (NMT2) ANNOUNCED TODAY THE APPROVAL BY THE U.S. FDA OF ITS IDE OF NOBLESTITCH™ EL FOR PFO CLOSURE FOR REDUCTION OF RECURRENT ISCHEMIC STROKE FOUNTAIN VALLEY, Calif., Sept. 5, 2019 /PRNewswire/ — Nobles Medical Technologies II has received approval from the U.S. Food and Drug Administration (USFDA) […]
Shockwave Receives FDA Breakthrough Device Designation for the Coronary IVL System
SANTA CLARA, Calif., Sept. 03, 2019 (GLOBE NEWSWIRE) — Shockwave Medical, Inc., a pioneer in the development and commercialization of Intravascular Lithotripsy (IVL) to treat complex calcified cardiovascular disease, today announced that the company has received Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) for its Shockwave […]
Concept Medical Inc. Granted ‘Breakthrough Device Designation’ From the FDA for Its MagicTouch AVF Sirolimus Coated Balloon
TAMPA, Fla.–(BUSINESS WIRE)–Concept Medical Inc. (CMI) has been granted “Breakthrough Device Designation” from the U.S. Food and Drug Administration (FDA) for MagicTouch AVF, its Sirolimus drug-coated balloon (DCB) catheter, for the treatment of stenotic lesions of Arteriovenous Fistulae or Arteriovenous graft in hemodialysis treatment of renal failure. The FDA received Concept […]