AstraZeneca today announced that the US Food and Drug Administration (FDA) has granted Fast Track designation for the development of FARXIGA (dapagliflozin) to delay the progression of renal failure and prevent cardiovascular (CV) and renal death in patients with chronic kidney disease (CKD). The FDA’s Fast Track program is designed […]
Regulatory
First-ever: FDA Clears Biobeat’s Wearable Watch and Patch for Non-invasive Cuffless Monitoring of Blood Pressure
TEL AVIV, Israel, Aug. 26, 2019 /PRNewswire/ — Biobeat, a bio-medical technology company developing advanced sensing and remote monitoring solutions for patients, announced today that the U.S. Food and Drug Administration (FDA) has granted a 510K clearance for its patch and watch for measurement of blood pressure, oxygenation and heart rate in hospitals, clinics, long-term […]
Miracor Medical Granted FDA Breakthrough Device Designation for the PiCSO Impulse System
AWANS, Belgium–(BUSINESS WIRE)–Miracor Medical SA (Miracor Medical) has been granted Breakthrough Device Designation by the U.S. Food and Drug Administration (FDA) for its PiCSO® Impulse System for treatment of STEMI patients. The FDA Breakthrough Device designation is intended to speed time to market for treatments of life-threatening or irreversibly debilitating diseases […]
FDA approves new device to improve symptoms in patients with advanced heart failure
SILVER SPRING, Md., Aug. 16, 2019 /PRNewswire/ — The U.S. Food and Drug Administration today approved the Barostim Neo System for the improvement of symptoms in patients with advanced heart failure who are not suited for treatment with other heart failure devices, such as cardiac resynchronization therapy. The FDA gave the device […]
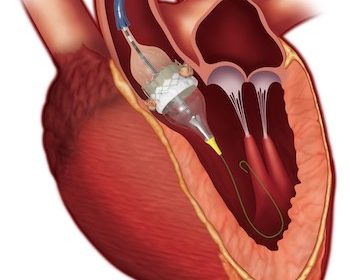
Medtronic Evolut TAVR System Receives Expanded Indication to Treat Symptomatic Severe Aortic Stenosis Patients at Low Risk for Surgical Mortality
DUBLIN, Aug. 16, 2019 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) today announced U.S. Food and Drug Administration (FDA) approval of the Evolut™ Transcatheter Aortic Valve Replacement (TAVR) system for patients with symptomatic severe native aortic stenosis who are at a low risk of surgical mortality. The low-risk patient population is […]
Edwards SAPIEN 3 TAVR Receives FDA Approval For Low-Risk Patients
IRVINE, Calif., Aug. 16, 2019 /PRNewswire/ — Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced U.S. Food and Drug Administration (FDA) approval to expand use of the Edwards SAPIEN 3 and SAPIEN 3 Ultra transcatheter heart valve systems to the treatment […]
FDA Grants Breakthrough Device Designation to Ascyrus Medical Dissection Stent (AMDS)
Ascyrus Medical receives Breakthrough Device Designation from the FDA for its Ascyrus Medical Dissection Stent (AMDS) to treat acute Type A aortic dissections. BOCA RATON, FLA. (PRWEB) AUGUST 14, 2019 Ascyrus Medical announced today that it has received Breakthrough Device Designation from the FDA for its Ascyrus Medical Dissection Stent (AMDS) […]
V-Wave’s Interatrial Shunt Receives FDA Breakthrough Device Designation for Heart Failure
CAESAREA, Israel, Aug. 15, 2019 /PRNewswire/ — V-Wave Ltd., a privately held medical device company developing novel implantable interatrial shunt devices, today announced that the U.S. Food and Drug Administration (FDA) has just granted the company a Breakthrough Device Designation for its interatrial shunt for Heart Failure (HF). V-Wave’s minimally invasive, implanted interatrial […]
DynoSense Corp. Announces FDA Approval of Its Cloud Based Vital Signs Measurement System
SAN JOSE, Calif., Aug. 13, 2019 /PRNewswire/ — DynoSense Corp. announced today that the U.S. Food and Drug Administration (FDA) has granted the company clearance for its patented Vital Signs Measuring System, the world’s first most integrated and cloud-based vital signs measuring and recording platform. Its unique worldwide patented and award-winning design is as simple […]
Windtree Announces FDA Fast Track Designation for Istaroxime
WARRINGTON, Pa., Aug. 13, 2019 /PRNewswire/ — Windtree Therapeutics, Inc. (OTCQB: WINT), a biotechnology and medical device company focused on developing drug product candidates and medical device technologies to address acute cardiovascular and pulmonary diseases, today announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track Designation for istaroxime for the […]