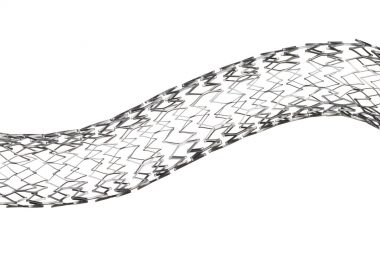
FRANKLIN LAKES, N.J., March 14, 2019 /PRNewswire/ — BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today announced the U.S. Food and Drug Administration has granted premarket approval for the Venovo™ venous stent, the first stent indicated to treat iliofemoral venous occlusive disease, which is obstructed or narrowed blood flow […]
Regulatory
FDA approves new indication for valve repair device to treat certain heart failure patients with mitral regurgitation
SILVER SPRING, Md., March 14, 2019 /PRNewswire/ — The U.S. Food and Drug Administration today approved a new indication for a heart valve repair device that is intended to reduce moderate-to-severe or severe mitral regurgitation, a leakage of blood backward through the mitral valve into the heart’s left atrium that can cause […]
ABBOTT RECEIVES FDA APPROVAL FOR EXPANDED INDICATION FOR MITRACLIP™ DEVICE
ABBOTT PARK, Ill., March 14, 2019 /PRNewswire/ — Abbott (NYSE: ABT) today announced it received approval from the U.S. Food and Drug Administration (FDA) for a new, expanded indication to its leading MitraClip™ device used to repair a leaky mitral valve without open-heart surgery. Supported by the results of the landmark COAPT™ Trial, […]
Espero BioPharma Announces Tecarfarin Receives FDA Orphan Drug Designation for Patients with End Stage Renal Disease and Atrial Fibrillation
JACKSONVILLE, Fla. and IRVINE, Calif., March 11, 2019 (GLOBE NEWSWIRE) — Espero BioPharma, Inc., a pharmaceutical company focused on the development of therapeutics for unmet needs in thrombosis and cardiac rhythm control, today announced that the U.S. Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) for tecarfarin […]
Water Street’s Partnership with Leading Medical Products Company Leads to FDA Approval and Launch of Ready-to-Use Cardiovascular Medicine Eptifibatide
CHICAGO, March 6, 2019 /PRNewswire/ — Water Street Healthcare Partners, a strategic investor focused exclusively on the health care industry, announced today that its development partnership with a leading medical products company has resulted in the U.S. Food and Drug Administration (FDA) approval and launch of the ready-to-use cardiovascular medication, eptifibatide. It is […]
FDA Grants Saranas De Novo Designation for the Early BirdTM Bleed Monitoring System
HOUSTON–(BUSINESS WIRE)–Saranas Inc., a medical device company with innovative technology for real-time detection and monitoring of internal bleeding during endovascular procedures, today announced that it has been granted de novo classification by the U.S. Food and Drug Administration (FDA) for the Early Bird Bleed Monitoring System. According to a recent […]
Medtronic Resolute(TM) Drug-Eluting Stent (DES) Platform Receives Expanded Indication for Treatment of Chronic Total Occlusion (CTO)
DUBLIN – February 26, 2019 – Medtronic plc (NYSE:MDT) today announced the U.S. Food and Drug Administration (FDA) approval of its Resolute Drug-Eluting Stent (DES) platform (including the Resolute Onyx(TM) and Resolute Integrity(TM) DES) for the treatment of patients with coronary artery disease who have de novo chronic total occlusion (CTO), a complex […]
ARCA Biopharma Announces FDA Agreement for a Single Phase 3 Clinical Trial to Support Approval for the First Genetically-Targeted Cardiovascular Drug
WESTMINSTER, Colo., Feb. 20, 2019 (GLOBE NEWSWIRE) — ARCA biopharma, Inc. (Nasdaq: ABIO), a biopharmaceutical company applying a precision medicine approach to developing genetically-targeted therapies for cardiovascular diseases, today announced that it has reached agreement with the U.S. Food and Drug Administration (FDA) regarding a Special Protocol Assessment (SPA) on the design of a […]
Edwards PASCAL Transcatheter System Receives CE Mark
IRVINE, Calif., Feb. 19, 2019 /PRNewswire/ — Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced the Edwards PASCAL transcatheter valve repair system has received CE Mark for the treatment of patients with mitral regurgitation. “Mitral valve disease is complex, varied and […]
Foldax, Inc. Gains Approval to Begin US Clinical Trials of the First and Only Biopolymer Heart Valve Platform
SALT LAKE CITY–(BUSINESS WIRE)–Foldax, Inc. today announced that the U.S. Food and Drug Administration (FDA) has granted IDE approval to begin an Early Feasibility Study of the Tria surgical aortic heart valve for the treatment of aortic valve disease. Using breakthrough LifePolymer™ – a proprietary advanced biopolymer material – and […]