WALTHAM, Mass.–(BUSINESS WIRE)–Corindus Vascular Robotics, Inc. (“Corindus” or the “Company”) (NYSE American: CVRS), a leading developer of precision vascular robotics, announced today it is seeking premarket clearance from the U.S. Food and Drug Administration (FDA) to use the CorPath GRX System in neurovascular intervention. CorPath GRX, the second generation robotic platform, received FDA clearance for percutaneous […]
Regulatory
Medtronic Receives FDA Approval on Expanded Indication for Pipeline Flex Embolization Device
DUBLIN – February 7, 2019 – Medtronic plc (NYSE:MDT) announced today that it has received U.S. Food and Drug Administration (FDA) approval on an expanded indication for its Pipeline(TM) Flex embolization device.1 Previously indicated for the endovascular treatment of adults with large or giant wide-necked intracranial aneurysms (IAs) in the internal carotid […]
Teleflex Announces U.S. FDA Premarket Approval of MANTA™ Vascular Closure Device
WAYNE, Pa.–(BUSINESS WIRE)–Teleflex Incorporated (NYSE: TFX), a leading global provider of medical technologies for critical care and surgery, has announced that it received premarket approval (PMA) from the U.S. Food and Drug Administration for the MANTA™ Vascular Closure Device – the first commercially available biomechanical vascular closure device designed specifically […]
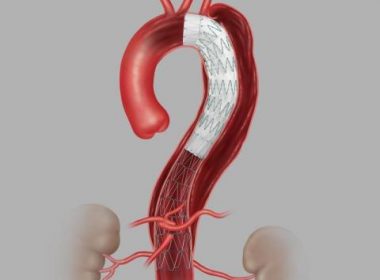
Cook Medical Receives U.S. FDA Approval for Aortic Dissection Device
BLOOMINGTON, Ind.–(BUSINESS WIRE)–Today, Cook Medical announced its recent approval from the U.S. FDA for its Zenith Dissection Endovascular System. The system, consisting of a proximal stent-graft component and a distal bare stent component, provides physicians a less invasive alternative to open surgery for repair of Type B dissections of the […]
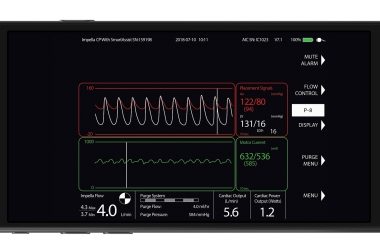
Impella Connect Achieves Regulatory Milestone
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ:ABMD) has achieved CE Mark for Impella Connect, the first-of-its kind cloud-based technology that enables secure, real-time, remote viewing of the Impella console for physicians and hospital staff from anywhere with Internet connectivity. European CE Mark adds to Impella Connect’s previous U.S. FDA PMA approval. Impella Connect uses […]
XableCath Crossing Catheters FDA Cleared
SALT LAKE CITY, Jan. 30, 2019 (GLOBE NEWSWIRE) — XableCath, Inc., announced that its XableCath™ blunt and abrasion tip catheters, were cleared by the FDA as Peripheral Crossing Catheters. The catheters have been safely and successfully used to cross challenging lesions in both arterial CTO’s and chronic obstructive venous lesions. […]
CorMatrix® Cardiovascular, Inc. receives FDA IDE approval to conduct the safety and feasibility clinical trial of the Cor™ TRICUSPID ECM® valve for pediatric and adult patients
ATLANTA, Jan. 24, 2019 /PRNewswire/ — CorMatrix® Cardiovascular, Inc. www.cormatrix.com, a leading developer of regenerative cardiovascular medical devices, today announced FDA approval of an early feasibility study IDE to evaluate the safety and feasibility of the Cor™ TRICUSPID ECM® cardiac valve for adults with endocarditis and for pediatric patients with congenital heart valve disease. The […]
Rebound Therapeutics Announces FDA Clearance of the Aurora Surgiscope System for Minimally Invasive Neurosurgery
IRVINE, Calif.–(BUSINESS WIRE)–Rebound Therapeutics® Corporation today announced FDA 510k clearance of their AURORA Surgiscope® System, the world’s first single-use disposable Neurosurgical Endoscope for minimally invasive access, visualization and illumination of the target neuro anatomy. The Aurora Surgiscope System consists of two components: the sterile, single use, neurological endoscope called the Aurora Surgiscope […]
Imperative Care Announces FDA Clearance of Initial Products
CAMPBELL, Calif.–(BUSINESS WIRE)–Imperative Care, Inc., a company singularly dedicated to finding answers to unsolved problems in stroke, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance of its first family of access catheters, designed to deliver interventional treatments during minimally invasive neurovascular procedures for aneurysms, stroke and other brain […]
Verily Study Watch Receives FDA 510(k) Clearance for ECG
In April of 2017, we launched Verily Study Watch, an investigational device for capturing health information from clinical research participants while serving as an easy-to-read watch for daily wear. Since then, Study Watch has been used by thousands of participants in clinical research studies run by Verily and through our partners, such […]