HOUSTON, TX, June 18, 2018 (GLOBE NEWSWIRE) — AngioSoma, Inc. (SOAN) is excited to announce that we have identified a regulatory firm who can assist taking our patented flagship drug, Liprostin™, through the FDA approval process. The regulatory firm will begin with a presubmission meeting with the FDA biologic group […]
Regulatory
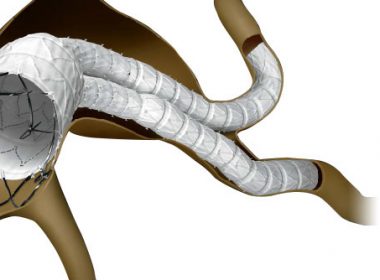
FDA Advisory Committee Votes in Favor of Cardinal Health’s INCRAFT® AAA Stent Graft System for the Endovascular Treatment of Infrarenal Abdominal Aortic Aneurysms
DUBLIN, Ohio, June 12, 2018 /PRNewswire/ — Cardinal Health (NYSE: CAH) today announced that the U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel of the Medical Devices Advisory Committee has provided a favorable recommendation on the premarket approval application for INCRAFT® AAA Stent Graft System (INCRAFT). The panel voted 11 to 4 in […]
LivaNova Receives FDA Clearance and Completes First Implant for its MEMO 4D Semi-rigid Mitral Annuloplasty Ring
LONDON–(BUSINESS WIRE)–LivaNova PLC (NASDAQ:LIVN) (“LivaNova” or the “Company”), a market-leading medical technology company, today announced it received FDA 510(k) clearance for its MEMO 4D® semi-rigid mitral annuloplasty ring and confirmed the first implantation of the device. MEMO 4D, LivaNova’s next-generation of the MEMO device family, now offers a broader range of […]
MicroVention Announces FDA Clearance For Thrombectomy Device
ALISO VIEJO, Calif., June 12, 2018 /PRNewswire/ — MicroVention, Inc. a U.S.-based subsidiary of Terumo and a global neurovascular company, announced today the FDA clearance of a new clinical indication for the SOFIA® Catheter (Soft TOrqueable catheter For Intracranial Access) to include contact aspiration technique for successful revascularization among patients with acute ischemic stroke, secondary to intracranial large […]
Correvio Provides U.S. Regulatory Update for BRINAVESS
VANCOUVER, June 11, 2018 /PRNewswire/ – Correvio Pharma Corp. (NASDAQ: CORV) (TSX: CORV), a revenue-generating, specialty pharmaceutical company focused on providing innovative, high-quality brands that meet the needs of acute care physicians and patients, today announced that it has received a response from the U.S. Food and Drug Administration (FDA) regarding the regulatory […]
Celixir announces US FDA approval of the IND application for cell therapy Heartcel
Stratford-upon-Avon, UK, 08 June 2018 – Celixir, a privately owned company discovering and developing life-saving advanced therapies, announces that the US Food and Drug Administration (FDA) has approved its Investigational New Drug application (IND) for HeartcelTM, its immuno-modulatory progenitor (iMP) cell therapy for the treatment of adult heart failure. Celixir announced […]
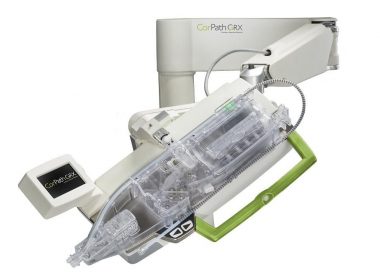
Corindus Announces Pharmaceutical and Medical Device Agency (PMDA) Approval of CorPath GRX System in Japan
WALTHAM, Mass.–(BUSINESS WIRE)–Corindus Vascular Robotics, Inc. [NYSE American: CVRS], a leading developer of precision vascular robotics, announced today that it received Pharmaceutical and Medical Device Agency (PMDA) Approval for commercialization of its CorPath® GRX System in Japan. Japan is one of the largest markets in the world for percutaneous coronary interventions […]
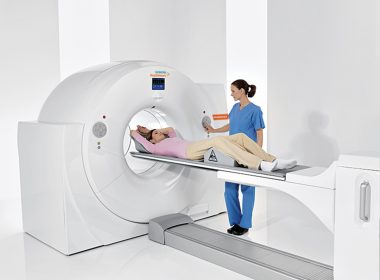
FDA Clears Biograph Vision PET/CT System From Siemens Healthineers
MALVERN, Pa.–(BUSINESS WIRE)–The Food and Drug Administration (FDA) has cleared the Biograph Vision, a new positron emission tomography/computed tomography system from Siemens Healthineers that delivers a new level of precision in PET/CT imaging. The Biograph Vision features new Optiso Ultra Dynamic Range (UDR) Detector Technology, which is based on silicon […]
FDA Classifies HeartWare(TM) HVAD(TM) Systems Unexpected Power Source Switching as Class I Recall
DUBLIN – June 1, 2018 – The United States Food and Drug Administration (FDA) has classified Medtronic plc’s (NYSE: MDT) recent voluntary urgent field action related to the HeartWare(TM) HVAD(TM) System unexpected power source switching as a Class I recall. Class I recalls describe situations where there is reasonable risk of […]
MicroVention® Announces FDA Approval For Neuro Stent Device
ALISO VIEJO, Calif., May 31, 2018 /PRNewswire/ — MicroVention, Inc., a U.S.-based subsidiary of Terumo and a global neurovascular company, announced today the FDA Premarket Approval (PMA) for the LVIS® and LVIS® Jr. stents for stent-assisted coil embolization of intracranial aneurysms. The LVIS® and LVIS® Jr. stents are the first and only stents PMA approved for […]