REDWOOD CITY, Calif., April 12, 2017 (GLOBE NEWSWIRE) — Biotricity, Inc. (OTCQB:BTCY), a medical diagnostic and consumer healthcare technology company dedicated to delivering innovative, biometric remote monitoring solutions, has filed for a second and final 510(k) for the hardware portion of its Bioflux solution with the U.S. Food and Drug Administration […]
Regulatory
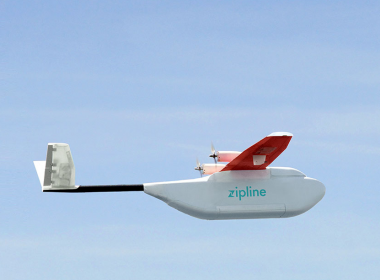
Coming to a Transplant Unit Near You: Drones Impact on the Delivery of Healthcare
BY KEN DROPIEWSKI The FAA predicts there will be 7 million drones registered in the US by 2020. While over half of the drones flying today belong to hobbyists, the rest are owned by private enterprise and various government agencies. However, despite their popularity, drones are a controversial tech. Opponents […]
Cordis, a Cardinal Health Company, introduces innovative products at EUROPCR 2017 to rapidly expand interventional cardiology offering
PCRonline/News/Industry Press Releases – 15 May, 2017 DUBLIN, Ohio. Cordis, Cardinal Health‘s interventional vascular business, announced today the commercial launch of three new products that further enhance its interventional cardiology product portfolio in Europe, the Middle East, and Africa (EMEA). The addition of the Cordis RAILWAY Sheathless Access System as well […]
Cardiovascular Systems Snags FDA Nod for Its Diamondback 360 Coronary Orbital Atherectomy System (OAS)
Cardiovascular Systems, Inc. Receives Approval for the Diamondback 360® Coronary Orbital Atherectomy System (OAS) Micro Crown in the United States OAS Micro Crown Approved to Treat Severely Calcified Coronary Lesions Only Atherectomy Device Designed to Both Pilot Tight Lesions and Treat Up to 4mm Vessels with a Single Device ST. […]
Surgery Without the Knife: Mirabilis Medical Announces European Approval for Non-Invasive Uterine Fibroid Treatment
BOTHELL, Wash., March 23, 2017 /PRNewswire/ — Mirabilis Medical, a Seattle-area developer of advanced medical technology for non-invasive surgery, announced today CE Mark authorization for marketing of the Mirabilis System for treatment of uterine fibroids throughout the European Union. The company simultaneously announced that it had received approval from the US […]
AngioDynamics Receives CE Mark Certification for The Solero Microwave Tissue Ablation System
ALBANY, N.Y., March 14, 2017 (GLOBE NEWSWIRE) — AngioDynamics (Nasdaq:ANGO), a leading provider of innovative, minimally invasive medical devices for vascular access, peripheral vascular disease and oncology/surgery, today announced it has received CE Mark certification for the Solero Microwave Tissue Ablation (MTA) System. The Solero MTA System and Accessories are […]
Humacyte Receives FDA Regenerative Medicine Advanced Therapy (RMAT) Expedited Review Designation for HUMACYL® in Vascular Access for Hemodialysis
Press Release RESEARCH TRIANGLE PARK, N.C. — Humacyte, an innovator in biotechnology and regenerative medicine, announced today that the U.S. Food and Drug Administration (FDA) has granted HUMACYL®, its investigational human acellular vessel (HAV), the Regenerative Medicine Advanced Therapy (RMAT) designation. This designation means that the FDA will help facilitate […]
Aegis Medical Innovations Announces FDA Approval of Clinical Trial
VANCOUVER, British Columbia–(BUSINESS WIRE)–Aegis Medical Innovations Inc. (Aegis) today announced that it has received Investigational Device Exemption approval from the U.S. FDA to initiate a clinical trial in the U.S. for its medical device called the Sierra Ligation System (Sierra). Aegis developed the Sierra technology in partnership with the Mayo […]
BioStable Science & Engineering Wins FDA Nod for Its HAART 300 Aortic Annuloplasty Device
BioStable Science & Engineering Announces FDA Clearance of the HAART™ 300 Aortic Annuloplasty Device AUSTIN, Texas–(BUSINESS WIRE)–BioStable Science & Engineering, Inc. announced today it has received FDA market clearance for the HAART 300 Aortic Annuloplasty Device, the first commercially available internal annuloplasty device designed for aortic valve repair. BioStable expects […]
FDA Green Lights Medtronic (MDT)’s CoreValve Evolut Pro Transcatheter Valve With Advanced Sealing
Press Release Medtronic Receives FDA Approval for CoreValve(TM) Evolut(TM) Pro Transcatheter Valve with Advanced Sealing DUBLIN – March 22, 2017 – Medtronic plc (NYSE:MDT) today announced U.S. Food and Drug Administration (FDA) approval and U.S. launch of the CoreValve(TM)Evolut(TM) PRO valve for the treatment of severe aortic stenosis for symptomatic […]