LATHAM, N.Y.–(BUSINESS WIRE)–AngioDynamics, Inc. (NASDAQ: ANGO), a leading and transformative medical technology company focused on restoring healthy blood flow in the body’s vascular system, expanding cancer treatment options and improving patient quality of life, today announced that the United States Food and Drug Administration (FDA) has cleared the Auryon XL Catheter, […]
Regulatory
Nectero Medical Announces Initiation of Phase II/III Clinical Trial of the Nectero EAST® System for Treatment of Small- to Medium-Sized Abdominal Aortic Aneurysms
TEMPE, Ariz.–(BUSINESS WIRE)– #AbdominalAorticAneurysm–Nectero Medical, a clinical-stage biotechnology company pioneering novel therapies to treat aneurysmal disease and improve patients’ lives, today announced initiation of a Phase II/III clinical trial (stAAAble) to investigate the safety and efficacy of the Nectero Endovascular Aneurysm Stabilization Treatment (Nectero EAST®) System in patients with infrarenal abdominal aortic aneurysms (AAAs), maximum diameter 3.5 – 5.0cm. The Nectero EAST System is a single-use,
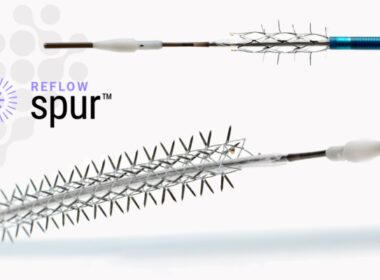
Reflow Medical Receives CE Mark for Bare Temporary Spur Stent System for Treating de novo or Restenotic Below-the-Knee (BTK) Lesions
San Clemente, CA—Jan. 15, 2024 – Reflow Medical, Inc., a developer of innovative medical devices focused on cardiovascular disease, announces it has received CE (Conformité Européenne) Mark certification in the European Union for the Bare Temporary Spur Stent System. The device is intended to treat de novo or restenotic lesions […]
Endovascular Engineering’s Hēlo™ Thrombectomy System Receives IDE Approval to Start ENGULF Pivotal Trial for the Treatment of Pulmonary Embolism
MENLO PARK, Calif., Jan. 11, 2024 /PRNewswire/ — Endovascular Engineering (“E2”), a medical device company focused on the development and deployment of groundbreaking clot removal technologies that target venous thromboembolism (VTE), announced today FDA Investigational Device Exemption…
Arch Biopartners Receives Health Canada Approval to Conduct Phase II Trial for LSALT Peptide Targeting Cardiac Surgery Associated-Acute Kidney Injury
TORONTO, Jan. 10, 2024 (GLOBE NEWSWIRE) — Arch Biopartners Inc., (“Arch” or the “Company”) (TSX Venture: ARCH and OTCQB: ACHFF), announced today that it has received a No Objection Letter from Health Canada to conduct a Phase II trial for LSALT peptide, targeting the prevention and treatment of cardiac surgery-associated […]
Biosense Webster Announces Regulatory Approval of VARIPULSE™ Pulsed Field Ablation (PFA) Platform in Japan
First regulatory approval for the first-of-its-kind, fully-integrated PFA system with a simple and reproducible workflow Integrated with the world’s leading CARTO™ 3D cardiac mapping system for the treatment of symptomatic drug refractory recurrent paroxysmal atrial fibrillation (AFib)…
American Medical Association (AMA) Grants Cardio Diagnostics Holdings, Inc.’s AI-Powered Coronary Heart Disease Detection Test, PrecisionCHD, a Dedicated CPT PLA Reimbursement Code
CHICAGO–(BUSINESS WIRE)–Cardio Diagnostics Holdings, Inc. (Nasdaq: CDIO), an AI-driven precision cardiovascular medicine company, today announced that the American Medical Association (AMA) has assigned a dedicated Current Procedural Terminology (CPT®) Proprietary Laboratory Analysis (PLA), 0440U, for the company’s AI-driven coronary heart disease (CHD) detection test, PrecisionCHD. Receipt of this new CPT […]
Pulse Biosciences Files 510(k) Submission with U.S. FDA for its CellFX® nsPFA™ Cardiac Clamp
HAYWARD, Calif.–(BUSINESS WIRE)–Pulse Biosciences, Inc. (Nasdaq: PLSE), a company primarily focused on leveraging its novel and proprietary CellFX Nanosecond Pulsed Field Ablation (nsPFA) technology for the treatment of atrial fibrillation, today announced the filing of a premarket notification 510(k) to the U.S. Food and Drug Administration (FDA) for its novel […]
Renibus Therapeutics Announces Breakthrough Therapy Designation from US FDA for RBT-1 for the Reduction in Risk of Post-Operative Complications in Patients Undergoing Cardiothoracic Surgery
SOUTHLAKE, Texas, July 11, 2023 /PRNewswire/ — Renibus Therapeutics, Inc., (“Renibus”), a clinical-stage biopharmaceutical company focusing on the prevention and treatment of cardio-renal diseases, today announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Therapy designation to RBT-1 for the reduction in risk of post-operative complications in patients undergoing cardiothoracic […]
MedRhythms Announces FDA Listing of InTandem™ (MR-001) to Improve Walking and Ambulation in Adults with Chronic Stroke
Stroke is a leading cause of long-term disability, and over half of people aged 65 or older who have had a stroke experience reduced mobility and gait deficits PORTLAND, Maine, July 6, 2023 /PRNewswire/ — MedRhythms today announced that MR-001, the company’s evidence-based neurorehabilitation system to improve walking and ambulation in adults with chronic […]