SAINT PAUL, Minn.–(BUSINESS WIRE)–Aria CV, Inc., a developer of medical devices treating Pulmonary Arterial Hypertension (PAH), today announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for the Aria CV Pulmonary Hypertension System (Aria CV PH System). The Breakthrough Devices Program (BDP) is intended to […]
Rhythm
CARMAT Announces FDA Full Approval to Initiate US Clinical Feasibility Study of Its Total Artificial Heart
CARMAT has responded to all remaining questions from the conditional approval Number of subjects to be enrolled in the study extended to 10 patients PARIS–(BUSINESS WIRE)–Regulatory News: CARMAT (Paris:ALCAR) (FR0010907956, ALCAR), the designer and developer of the world’s most advanced total artificial heart, aiming to provide a therapeutic alternative for […]
Imricor Announces First Cases Successfully Performed at Heart Center Dresden
MINNEAPOLIS & DRESDEN, Germany–(BUSINESS WIRE)–Imricor Medical Systems, Inc. (Company or Imricor) (ASX:IMR) is pleased to announce the first procedures using the Company’s products following CE mark of the Vision-MR Ablation Catheter have been performed at the Heart Center Dresden. Three procedures were successfully performed over two days by Dr. Christopher Piorkowski and Dr. […]
CardioFocus Announces U.S. FDA PMA Supplement Submission For The Breakthrough HeartLight X3 Endoscopic Ablation System
MARLBOROUGH, Mass., Feb. 4, 2020 /PRNewswire/ — CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for atrial fibrillation (AFib), today announced that the company has submitted a pre-market approval (PMA) supplement to the U.S. Food and Drug Administration (FDA) for the HeartLight X3 Endoscopic Ablation System for the treatment of AFib. This […]
ABBOTT ANNOUNCES FIRST-OF-ITS-KIND TRIAL TO ASSESS NEW THERAPY OPTION FOR PEOPLE AT RISK OF STROKE
– The CATALYST trial will examine Abbott’s Amplatzer™ Amulet™ device compared to non-vitamin K oral anticoagulants, the current standard in attempting to lower stroke and bleeding risks for patients with atrial fibrillation ABBOTT PARK, Ill., Feb. 3, 2020 /PRNewswire/ — Abbott (NYSE: ABT) today announced that the U.S. Food and Drug Administration […]
Hackensack Meridian Hackensack University Medical Center First Hospital in Nation to Implant World’s First Heart Failure Neuromodulation Device
BAROSTIM NEO™ provides effective treatment for heart failure patients HACKENSACK, N.J., Feb. 3, 2020 /PRNewswire/ — Hackensack Meridian Hackensack University Medical Center announced today that it is the first hospital in the nation to successfully implant the world’s first heart failure neuromodulation device, BAROSTIM NEO™. This innovative technology is an effective alternative for […]
Medtronic Receives CE Mark for Cobalt™ and Crome™ Portfolio of BlueSync™-Enabled Implantable Defibrillators, Cardiac Resynchronization Therapy-Defibrillators
Next Generation Technology Includes Features that Automatically Adjust to Patient Needs, and Offers Physicians Heart Failure Diagnostic Insights DUBLIN, Jan. 30, 2020 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) today announced it has received CE (Conformité Européenne) Mark for its Cobalt™ and Crome™ portfolio of implantable cardioverter-defibrillators (ICD) and cardiac resynchronization therapy-defibrillators […]
Biofourmis Partners with ImagineMIC to Pioneer Collaborative Remote Patient Monitoring Model
Biofourmis’ artificial intelligence-powered RhythmAnalytics® to provide clinical decision support that IDs cardiac arrhythmias and other issues requiring intervention BOSTON, Jan. 30, 2020 /PRNewswire/ — Biofourmis, a fast-growing global leader in digital therapeutics that powers personally predictive care, and ImagineMIC™ announced a partnership agreement today to drive improved outcomes and lower healthcare costs for […]
Late-Breaking Clinical Study Presented at AF Symposium 2020 Evaluates Attune Medical’s ensoETM For Use During Cardiac Ablation Procedures
CHICAGO–(BUSINESS WIRE)–Chicago-based medical device firm Attune Medical announced the presentation of clinical results from the largest device study to date designed to evaluate the performance of its ensoETM patient temperature management device as an aid in the reduction of esophageal injury during cardiac ablation, a common treatment for atrial fibrillation. […]
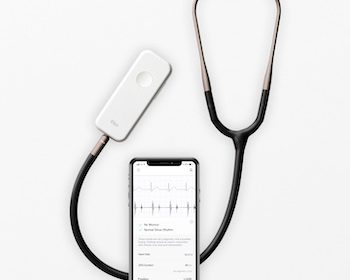
FDA Clears Eko’s AFib and Heart Murmur Detection Algorithms, Making It the First AI-Powered Stethoscope to Screen for Serious Heart Conditions
Eko’s algorithms alert clinicians to the presence of heart murmurs and atrial fibrillation (AFib) during the physical exam, converting the classic stethoscope into a powerful early detection tool SAN FRANCISCO–(BUSINESS WIRE)–Eko, a digital health company applying artificial intelligence (AI) in the fight against heart disease, announced today that the U.S. […]