Comprehensive analysis of real-world data shows significant clinical and medico-economic advantages of Implicity’s universal, alert-based remote monitoring platform CAMBRIDGE, Mass., April 24, 2025 /PRNewswire/ — IMPLICITY®, a leader in remote patient monitoring and cardiac data…
Rhythm
OMRON Healthcare Donates 4,000 Blood Pressure Monitors and Advances AFib Screening with AI-based Intellisense AFib Technology
– Intellisense AFib Technology Able to Detect Possible Atrial Fibrillation with Every Measurement; May 17 Marks World Hypertension Day during May Measurement Month – KYOTO, Japan, April 24, 2025 /PRNewswire/ — OMRON Healthcare Co., Ltd. (Head Office: Muko, Kyoto Prefecture; hereinafter…
XYRA Announces the Issuance of a Fifth New US Patent in a Series of Patents Which Protect the Use of Budiodarone in the Management of Atrial Fibrillation till after 2040
Budiodarone shown in Phase 2 to reduce atrial fibrillation symptoms and long episodes of atrial fibrillation (LEAF) FDA gives guidance on steps necessary to gain approval and incorporate wearable AF monitoring devices into label for budiodarone Five granted US patents align with FDA’s…
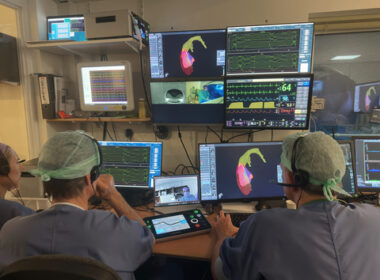
Stereotaxis to Feature First-Ever Live Demo of GenesisX Robotic System at HRS 2025
ST. LOUIS, April 21, 2025 (GLOBE NEWSWIRE) — Stereotaxis (NYSE: STXS), a pioneer and global leader in surgical robotics for minimally invasive endovascular intervention, announced today it will host a live demonstration of the GenesisX Robotic System at this year’s Heart Rhythm Symposium (HRS), taking place April 24 – 27 […]
CardioFocus to Showcase PFA Portfolio at Heart Rhythm 2025
Advancing PFA through a science-led, portfolio-driven approach to innovation MARLBOROUGH, Mass.–(BUSINESS WIRE)–CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for cardiac arrhythmias, is excited to announce its featured presence at Heart Rhythm 2025 in San Diego, where the company will showcase the latest advancements and clinical progress […]
Pulse Biosciences’ Nanosecond PFA Technology to be Featured at The Heart Rhythm Society 2025 Annual Meeting
Nanosecond PFA 360° Cardiac Catheter for AF ablation to be highlighted in presentations and in a live case transmission HAYWARD, Calif.–(BUSINESS WIRE)–Pulse Biosciences, Inc. (Nasdaq: PLSE), a company leveraging its novel and proprietary Nanosecond Pulsed Field Ablation™ (nanosecond PFA or nsPFA™) technology, today announced that its Nanosecond PFA technology will […]
Imricor Commences VISABL-VT Trial
MINNEAPOLIS–(BUSINESS WIRE)–Imricor Medical Systems, Inc. (Company or Imricor) (ASX: IMR) is pleased to announce that it has commenced the VISABL-VT clinical trial by completing the first-in-human ventricular ablation guided by real-time MRI with the Company’s NorthStar Mapping System. The procedure was performed by the team at the Amsterdam University Medical Centre (AUMC), ranked […]
Orchestra BioMed Announces AVIM Therapy-Focused Satellite Symposium at HRS 2025 Annual Meeting
NEW HOPE, Pa., April 23, 2025 (GLOBE NEWSWIRE) — Orchestra BioMed Holdings, Inc. (Nasdaq: OBIO, “Orchestra BioMed” or the “Company”), a biomedical company accelerating high-impact technologies to patients through risk-reward sharing partnerships, today announced it will host an industry-sponsored satellite symposium at the Heart Rhythm Society (“HRS”) 2025 Annual Meeting, taking place April 24–27, 2025, in San Diego, California featuring recent advancements in the Company’s atrioventricular interval modulation (“AVIM”) therapy program. The April 25th, 6:45 am PT symposium titled “The Future of Cardiac Pacing: Unlocking the Potential of Atrioventricular Interval Modulation (AVIM) Therapy” will convene leading electrophysiologists, hypertension and heart failure specialists to discuss the unmet need in hypertension, AVIM therapy mechanism of action, and growing body of clinical evidence supporting this novel therapy for the treatment of patients with uncontrolled hypertension who have increased cardiovascular risk with or without an indication for a pacemaker.
THE HEART RHYTHM SOCIETY ANNOUNCES FRAMEWORK FOR ESTABLISHING ATRIAL FIBRILLATION CENTERS OF EXCELLENCE AND KEY OPERATIONAL STANDARDS
WASHINGTON, April 23, 2025 /PRNewswire/ — Today, the Heart Rhythm Society (HRS) released a framework outlining criteria for establishing an Atrial Fibrillation (AF) Center of Excellence (CoE) and key operational standards to provide multidisciplinary care for AF patients. AF, the most…
FIELD MEDICAL ANNOUNCES FIRST-IN-HUMAN DATA FOR FOCAL PFA IN SCAR-RELATED VT AT HEART RHYTHM 2025
Eight presentations, including late-breaking science, live cases, and a landmark symposium CARDIFF-BY-THE-SEA, Calif., April 23, 2025 /PRNewswire/ — Field Medical Inc., a leader in cardiac pulsed field ablation (PFA) technology, announced today its FieldForce™ Ablation System will be…