– For people battling advanced heart failure, Abbott’s HeartMate 3 heart pump can now be implanted through an incision in the chest wall versus open heart surgery – New, less invasive technique provides cardiac surgeons with the ability to choose the optimal surgical method for their patients ABBOTT PARK, Ill., Jan. […]
Rhythm
AtriCure Announces Successful Completion of Patient Enrollment of aMAZE Clinical Trial and FDA Approval of Continued Access Protocol
MASON, Ohio–(BUSINESS WIRE)–AtriCure, Inc. (Nasdaq: ATRC), a leading innovator in treatments for atrial fibrillation (Afib) and left atrial appendage (LAA) management, today announced the successful completion of patient enrollment in the aMAZE™ clinical trial. In addition, the company announced that it has received approval from the Food and Drug Administration […]
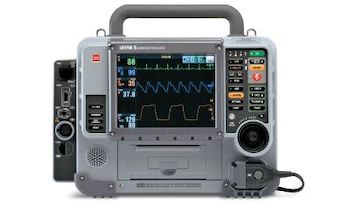
Stryker launches voluntary field action for specific units of the LIFEPAK® 15 monitor/defibrillator
KALAMAZOO, Michigan, USA, Dec. 27, 2019 /PRNewswire/ — Stryker announced today that the company is launching a voluntary field action on specific units of the LIFEPAK 15 monitor/defibrillators. The company is notifying a population of LIFEPAK 15 customers of an issue that may cause their devices to fail to deliver a defibrillation […]
Correvio Receives Complete Response Letter From U.S. FDA for Brinavess and Provides Update on Recent Events
NASDAQ: CORV TSX: CORV VANCOUVER, Dec. 24, 2019 /PRNewswire/ – Correvio Pharma Corp. (NASDAQ: CORV) (TSX: CORV), a specialty pharmaceutical company focused on commercializing hospital drugs, today announced it has received a Complete Response Letter (CRL) from the U.S. Food and Drug Administration (FDA) regarding the New Drug Application (NDA) for Brinavess™ (vernakalant IV), an anti-arrhythmic […]
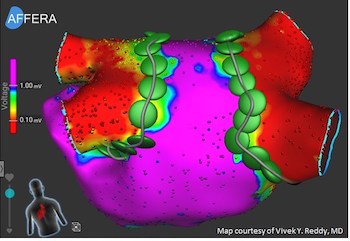
Affera Announces World’s First Successful Focal Pulsed Field Ablation in Patients
WATERTOWN, Mass., Dec. 19, 2019 /PRNewswire/ — Affera, Inc., a private medical device company focused on innovative cardiac arrhythmia treatment solutions, announced today that its focal Pulsed Field (“PF”) ablation technology, also known as Irreversible Electroporation (“IRE”), has been successfully used to treat 40 patients suffering from Atrial Fibrillation (“AFIB”). This multi-center […]
FDA Grants ‘Breakthrough’ Designation to Eko’s ECG-based Low Ejection Fraction Screening Algorithm, Designed to Improve Detection of Heart Failure
Developed in collaboration with Mayo Clinic, the algorithm would help healthcare providers detect heart failure during a standard physical exam San Francisco, CA, Dec. 18, 2019 (GLOBE NEWSWIRE) — Eko, a digital health company applying artificial intelligence (AI) in the fight against heart disease, today announced the U.S. Food and Drug Administration […]
ANI Pharmaceuticals Announces Launch of Bretylium Tosylate Injection, USP 50 mg/mL
BAUDETTE, Minn., Dec. 18, 2019 /PRNewswire/ — ANI Pharmaceuticals, Inc. (“ANI”) (Nasdaq: ANIP) today announced the launch of Bretylium Tosylate Injection USP, 50 mg/mL. Bretylium Tosylate is a class III antiarrhythmic medication approved for the treatment of ventricular fibrillation and life-threatening ventricular arrhythmias such as ventricular tachycardia. ANI estimates that the current annual U.S. […]
iRhythm Announces Participation in GUARD-AF Study Focused on Undiagnosed Atrial Fibrillation
SAN FRANCISCO, Dec. 16, 2019 (GLOBE NEWSWIRE) — iRhythm Technologies, Inc. (NASDAQ: IRTC) today announced its participation in a new, randomized, controlled study, GUARD-AF (ReducinG stroke by screening for UndiAgnosed atRial fibrillation in elderly inDividuals), sponsored by the Bristol-Myers Squibb-Pfizer Alliance (the Alliance). The study seeks to determine if earlier detection of atrial […]
Adagio Medical Announces First Patient Enrollment in its iCLAS™ U.S. IDE Clinical Trial Evaluating Ultra-Low Temperature Cryoablation for Persistent Atrial Fibrillation
LAGUNA HILLS, Calif., Dec. 16, 2019 /PRNewswire/ — Adagio Medical, Inc. (Adagio) today announced it has enrolled the first patient in its iCLAS™ Investigational Device Exemption (IDE) trial. The FDA approved study aims to evaluate the safety and efficacy of Adagio’s ultra-low temperature intelligent Continuous Lesion Ablation System (iCLAS) in the treatment of persistent atrial fibrillation (PsAF). Data will […]
CardiaCare Awarded First Prize at the Innovation in Cardiovascular Interventions (ICI) Technology Parade in Tel Aviv, Israel
TEL AVIV, Israel, Dec. 16, 2019 /PRNewswire/ — CardiaCare, a startup developing wearable therapeutic devices for treating and monitoring AFib, announced today that it has been named the First Prize winner of the 2019 Innovation in Cardiovascular Interventions (ICI) Technology Parade in Tel Aviv. CardiaCare presented its innovative product, a wearable neuromodulation (nerve […]