DUBLIN, Oct. 29, 2019 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT), the global leader in medical technology, today announced it has received Breakthrough Device designation from the U.S. Food and Drug Administration (FDA) for the Medtronic Fully Implantable Left Ventricular Assist Device (LVAD) for patients with advanced heart failure, currently in development. […]
Rhythm
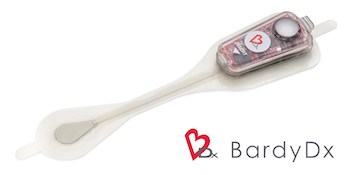
Bardy Diagnostics™ Wins the Remote Monitoring in Arrhythmias Digital Health Pitch Session at European Society of Cardiology Congress 2019 and Presents at UK Heart Rhythm Congress 2019
SEATTLE, Oct. 24, 2019 /PRNewswire/ — Bardy Diagnostics, Inc., (“BardyDx”), a leading provider of ambulatory cardiac monitoring technologies and custom data solutions, announced that it was selected as winner of the “Remote Monitoring in Arrhythmias” Technology and Innovation Pitch Session held as part of the Digital Health program at the European Society […]
Vygon Group Acquires Pilot, Specialist in ECG Central Venous Catheter Tip Location and Navigation Devices
Écouen, France, October 21, 2019 – Vygon Group, a specialist single-use medical devices group, today announces its acquisition of Italian firm Pilot, which specializes in ECG guidance devices. The financial terms of the agreement have not been disclosed. The aim of this transaction is to acquire the ECG location and navigation […]
Omron Healthcare Rolls Out Redesigned Line of Best-Selling Blood Pressure Monitors
LAKE FOREST, Ill., Oct. 17, 2019 /PRNewswire/ — Omron Healthcare, Inc., the number one doctor and pharmacist-recommended blood pressure brand, has rolled out newly redesigned models of its best-selling blood pressure monitors with new features. The redesigned upper arm and wrist blood pressure monitors build on the standard of quality Omron is […]
HeartBeam’s Breakthrough Technology to Bring Mobile Cardiovascular Diagnostic Services to Patients
MILL VALLEY, Calif., Oct. 16, 2019 /PRNewswire/ — Every year, nearly 800,000 Americans have a heart attack. Heart disease now accounts for one in four deaths, according to the Centers for Disease Control and Prevention. When patients have heart attack symptoms, the most important factor in a successful outcome is how quickly […]
BioSig Opens a Technology Development Office in Rochester, MN
Westport, CT, Oct. 15, 2019 (GLOBE NEWSWIRE) — BioSig Technologies, Inc. (NASDAQ: BSGM) (“BioSig” or “The Company”), a medical technology company developing a proprietary biomedical signal processing platform designed to improve signal fidelity and uncover the full range of ECG and intra-cardiac signals, today announced that the Company is opening […]
Kardium® announces successful results from the GLOBAL-AF study
VANCOUVER, British Columbia–(BUSINESS WIRE)–Kardium Inc. today announced that the Globe® mapping and ablation system has successfully demonstrated safety and efficacy in the GLOBAL-AF study. The results of the study were published in the Journal of Cardiovascular Electrophysiology. The study demonstrated a 76% percent freedom from atrial fibrillation at 12 months after a […]
Medtronic Initiates Worldwide Pivotal Study of a New Approach to Treating Dangerously Fast Heart Rhythms
Study to Evaluate Novel Implantable Defibrillator System with a Lead Placed Outside the Heart and Veins DUBLIN, Oct. 07, 2019 (GLOBE NEWSWIRE) — Medtronic plc (NYSE:MDT) today announced the start of a worldwide pivotal study evaluating its investigational Extravascular Implantable Cardioverter Defibrillator (EV ICD) system to treat dangerously fast heart rhythms. The […]
Preventice Solutions Receives Tender from UK Biobank for 3-Year, 30,000-Participant Cardiovascular Monitoring Study
MINNEAPOLIS, Oct. 2, 2019 /PRNewswire/ — Preventice Solutions announced today it received a tender from the UK Biobank to provide the BodyGuardian® MINI remote cardiac monitor for a cardiovascular monitoring study involving up to 30,000 participants. The study is designed to capture data on asymptomatic heart rhythm disturbances (arrhythmia), including atrial fibrillation (AF), […]
AdjuCor Announces First Successful In Vivo 60-Day Pre-Clinical Survival Study using its Cutting-edge Non-blood-contacting, Biventricular Cardiac Support Device.
After completing a 60-day pre-clinical study, Professor Stephen Wildhirt, MD, PhD, CEO at AdjuCor, is excited to report on its success. Apart from other achievements, the study confirmed the chronic physiologic acceptance of their non-blood-contacting biventricular cardiac support device by the body. AdjuCor has previously completed a successful 30-day in vivo study. […]