Company to spotlight ASSURE WCD performance and expanded clinical applicationsKIRKLAND, Wash., April 23, 2025 (GLOBE NEWSWIRE) — Kestra Medical Technologies, Ltd. (Nasdaq: KMTS) (“Kestra”), a wearable medical device and digital healthcare company, announced today it will exhibit at Heart Rhythm 2025, the annual meeting of the Heart Rhythm Society (HRS), taking place April 24-27 at the San Diego Convention Center. This marks Kestra’s first major industry showcase following its successful IPO earlier this year. Kestra will debut an immersive in-booth experience designed to bring the ASSURE® system to life—demonstrating how this innovative technology is redefining protection for patients at risk of sudden cardiac arrest. By combining lifesaving defibrillation therapy with intuitive, intelligent, and connected diagnostic and patient support capabilities, the ASSURE system is a key part of a broader vision for a holistic cardiac care ecosystem that supports patients and providers across the recovery journey. “Our presence at Heart Rhythm 2025 comes at a pivotal time for Kestra,” said Brian Webster, President and Chief Executive Officer of Kestra. “Following our IPO, we’re moving forward with increasing momentum—and this year’s HRS meeting is an opportunity to demonstrate how the ASSURE system goes beyond protection to offer a smarter, more connected recovery experience for both patients and care teams.” In addition to exhibiting, Kestra will also present new real-world clinical data highlighting the impact of the ASSURE system. Kestra is also proud to sponsor the Women in EP Luncheon for the fourth consecutive year—underscoring its ongoing commitment to leadership, innovation, and equity in cardiovascular care. About KestraKestra Medical Technologies, Ltd. is a commercial-stage wearable medical device and digital healthcare company focused on transforming patient outcomes in cardiovascular disease using monitoring and therapeutic intervention technologies that are intuitive, intelligent, and connected. For more information, please visit www.kestramedical.com. Media contactRhiannon Pickusrhiannon.pickus@kestramedical.com Investor contactNeil Bhalodkarneil.bhalodkar@kestramedical.com
Rhythm
FIELD MEDICAL CLOSES $40 MILLION SERIES A FINANCING TO REDEFINE PULSED FIELD ABLATION FOR VENTRICULAR TACHYCARDIA
CARDIFF-BY-THE-SEA, Calif., April 22, 2025 /PRNewswire/ — Field Medical Inc., a pioneer in cardiac pulsed field ablation (PFA) technology, today announced the successful closing of $40 million in Series A financing. The round includes $20 million in new capital and the conversion of $20…
The Future of AI & Electrophysiology Takes Center Stage at Heart Rhythm 2025 with Vektor Medical
SAN DIEGO–(BUSINESS WIRE)–Vektor Medical, a leader in non-invasive, AI-powered arrhythmia analysis technology, will showcase its transformative vMap technology at Heart Rhythm 2025, taking place April 25–27 in San Diego. Through live demonstrations at adjacent booths (#1523 and #1622), scientific abstract presentations, and a highly anticipated Rhythm Theater session featuring leading […]
CathVision to Exhibit the Latest Advancements in Electrophysiology at Heart Rhythm Society 2025 in San Diego
COPENHAGEN, Denmark, April 21, 2025 /PRNewswire/ — CathVision, a leader in advanced cardiac electrophysiology (EP) technology, is pleased to announce its participation and exhibition at the Heart Rhythm Society (HRS) 2025 Annual Scientific Sessions at the San Diego Convention Center in…
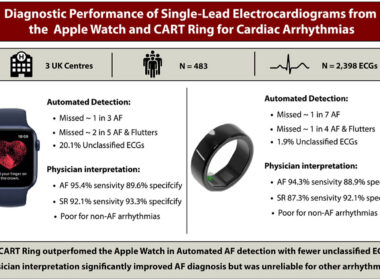
UK Clinical Study Finds Sky Labs’ Smart Ring ‘CART-I’ More Sensitive Than Apple Watch for AFib Detection
Published in the international journal Heart Rhythm O2, the clinical study demonstrates that CART-I achieved 84.6% sensitivity – higher than Apple Watch’s 69.1% Sky Labs also recognized with Korea’s top tech honor: IR52 Jang Young-sil Award – Prime Minister’s Award SEOUL, South Korea,…
Elutia Initiates EluPro™ Registry Study Designed to Generate Evidence Supporting the Use of EluPro in Real-World Clinical Practice
— Integration of clinical and patient-reported outcomes expected to further differentiate EluPro’s utility in cardiac implantable electronic device (CIED) procedures — SILVER SPRING, Md., April 21, 2025 (GLOBE NEWSWIRE) — Elutia Inc. (Nasdaq: ELUT) (“Elutia” or the “Company”), a pioneer in drug-eluting biomatrix technologies, today announced the initiation of an EluPro™ clinical study designed to collect patient outcome data in real-world clinical practice. EluPro, the first and only FDA-cleared antibiotic-eluting bioenvelope designed for use with cardiac implantable electronic devices (CIEDs) and neurostimulators, was commercially launched earlier this year. The first patient was enrolled at UC San Diego Health. “Every innovation we pursue is driven by a commitment to improving patient care,” said Kimberly Mulligan, PhD, Vice President and General Manager of Cardiovascular at Elutia. “With EluPro, we combined trusted antibiotics with a soft, regenerative biomatrix to protect the implant, facilitate implantation, and support healing. This study will allow us to collect data on these differentiating characteristics in real-world practice.” The multi-center clinical study is a prospective, post-market study designed to evaluate the use of EluPro in standard clinical practice and its performance across a diverse population of patients undergoing CIED implantation. Data on clinical and patient-reported outcomes will be collected, which will include assessments of key complications of interest following CIED implantation – such as infection, hematoma, lead dislodgement, device migration or erosion, and implant site complications. The study plans to enroll 100 patients, who will be followed for 12 months after device implantation. Each year, more than 600,000 CIEDs are implanted in the U.S., with overall complication rates up to 5-7%, including infections linked to higher morbidity and mortality. EluPro is cleared for use across all major CIED brands including pacemakers and implantable defibrillators, as well as for a wide range of neurostimulation devices. Unlike synthetic alternatives, EluPro addresses this critical need by combining the antibiotics rifampin and minocycline with a soft, regenerative biomatrix that promotes healing and helps reduce other complications, such as migration and erosion. The CIED protection market is valued at $600 million in the U.S. To learn more, visit www.elutia.com/products/elupro/. About Elutia Elutia develops and commercializes drug-eluting biomatrix products to improve compatibility between medical devices and the patients who need them. With a growing population in need of implantable technologies, Elutia’s mission is humanizing medicine so patients can thrive without compromise. For more information, visit www.Elutia.com. Forward Looking Statements This press release contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements can be identified by words such as “projects,” “may,” “will,” “could,” “would,” “should,” “believes,” “expects,” “anticipates,” “estimates,” “intends,” “plans,” “potential,” “promise” or similar references to future periods. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including any statements and information concerning the EluPro Registry Study, including the timing and anticipated data, and the value of the CIED protection market. These forward-looking statements are based on our management’s beliefs and assumptions and on information currently available to us. Such beliefs and assumptions may or may not prove to be correct. Additionally, such forward-looking statements are subject to a number of known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied in the forward-looking statements, including, but not limited to the following: our ability to successfully commercialize, market and sell our newly approved EluPro product; our ability to continue as a going concern; our ability to achieve or sustain profitability; the risk of product liability claims and our ability to obtain or maintain adequate product liability insurance; our ability to defend against the various lawsuits and claims related to our recalled FiberCel and other viable bone matrix products and avoid a material adverse financial consequence from those lawsuits and claims; the continued and future acceptance of our products by the medical community; our ability to enhance our products, expand our product indications and develop, acquire and commercialize additional product offerings; our dependence on our commercial partners and independent sales agents to generate a substantial portion of our net sales; our dependence on a limited number of third-party suppliers and manufacturers, which, in certain cases are exclusive suppliers for products essential to our business; our ability to successfully realize the anticipated benefits of the November 2023 sale of our Orthobiologics business; physician awareness of the distinctive characteristics, benefits, safety, clinical efficacy and cost-effectiveness of our products; our ability to compete against other companies, most of which have longer operating histories, more established products and/or greater resources than we do; pricing pressure as a result of cost-containment efforts of our customers, purchasing groups, third-party payors and governmental organizations could adversely affect our sales and profitability; our ability to obtain regulatory approval or other marketing authorizations by the FDA and comparable foreign authorities for our products and product candidates; and our ability to obtain, maintain and adequately protect our intellectual property rights; and other important factors which can be found in the “Risk Factors” section of Elutia’s public filings with the Securities and Exchange Commission (“SEC”), including Elutia’s Annual Report on Form 10-K for the year ended December 31, 2024, as such factors may be updated from time to time in Elutia’s other filings with the SEC, accessible on the SEC’s website at www.sec.gov and the Investor Relations page of Elutia’s website at https://investors.elutia.com. Because forward-looking statements are inherently subject to risks and uncertainties, you should not rely on these forward-looking statements as predictions of future events. Any forward-looking statement made by Elutia in this press release is based only on information currently available and speaks only as of the date on which it is made. Except as required by applicable law, Elutia expressly disclaims any obligations to publicly update any forward-looking statements, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise. Investors:Matt SteinbergFINN Partnersmatt.steinberg@finnpartners.com This press release was published by a CLEAR® Verified individual.
Adagio Medical Holdings, Inc. Receives FDA Breakthrough Device Designation for the vCLASTM Cryoablation System
Agency’s Breakthrough Device Designation Program Allows for Priority Review of Ablation Technology for Ventricular Tachycardia LAGUNA HILLS, Calif.–(BUSINESS WIRE)–Adagio Medical Holdings, Inc. (Nasdaq: ADGM) (“Adagio” or “the Company”), a leading innovator in catheter ablation technologies for the treatment of cardiac arrhythmias, today announced that it has received Breakthrough Device designation […]
LUMA Vision Receives FDA Clearance for VERAFEYE 2D/4D Cardiac Visualization Platform
Real-Time, 360-Degree Imaging Enhances Precision and Confidence in Cardiac Procedures DUBLIN, April 16, 2025 /PRNewswire/ — LUMA Vision Ltd., an innovative leader in advanced cardiac imaging and navigation, today announced the FDA clearance of its VERAFEYE™ Visualization Platform. This…
Catheter Precision, Inc. Receives Notice of First Patent Allowance in the US for LockeT Product Line
Fort Mill, S.C., April 14, 2025 (GLOBE NEWSWIRE) — Catheter Precision, Inc. (VTAK – NYSE/American), a US based medical device company focused on developing technologically advanced products for the cardiac electrophysiology market announced that it has received a notice of allowance from the US Patent Office of its first patent in the US for its LockeT product, filed in December 2022, for its design of a surgical closure device for orthoscopic entry wounds.
Merit Medical Launches the Ventrax™ Delivery System
Novel all-in-one retrograde aortic access delivery system supports streamlined treatment of ventricular tachycardiaSOUTH JORDAN, Utah, April 09, 2025 (GLOBE NEWSWIRE) — Merit Medical Systems, Inc. (NASDAQ: MMSI), a global leader in healthcare technology, today announced the US commercial release of its Ventrax Delivery System. The new delivery system is Merit’s latest addition to its growing electrophysiology (EP) and cardiac rhythm management (CRM) portfolio. The portfolio provides a unique selection of solutions to improve cardiac interventions, including the HeartSpan®, Worley™, Prelude SNAP™, and SafeGuard Focus® product lines. Ventrax is intended to facilitate placement of devices used in ablation procedures commonly performed to treat an abnormally fast heartbeat known as ventricular tachycardia (VT). Ventricular arrhythmias, which include VT, are believed to cause approximately three out of four sudden cardiac deaths, which result in an estimated 184,000–450,000 lives lost in the United States each year.¹ The all-in-one device provides a streamlined pathway for ablation catheters to enter the left ventricle through the aorta, an approach known as retrograde aortic access. “For the electrophysiologist, using retrograde aortic access was always a risk-benefit decision, and the community has needed a tool that would make that decision easier,” said Albert Sun, MD, electrophysiologist at Duke Health in Durham, NC, and whose advice was instrumental in Merit’s development of the Ventrax system. Retrograde aortic access can be useful to reach certain areas of the ventricle compared to traditional methods, such as transseptal puncture. Using this approach, these areas of the left ventricle are more accessible,² supporting targeted treatment. “The new retrograde delivery system provides access to the left ventricle, allowing for the exchange of catheters to diagnose, map, or treat VT,” said Jason Koontz, MD, PhD, electrophysiologist at Duke Health and product development consultant to Merit. “This adds to our tools as we work to deliver the best possible outcomes for our patients.” Learn more about the Ventrax Delivery System Key features of the Ventrax Delivery System include a 95-cm sheath designed to access desired target locations. An ultralow-profile transition between its sheath and pigtail-dilator offers smooth insertion, and an angled tip enhances the reach of an ablation catheter. VT ablation is one of the fastest growing areas in electrophysiology. Many physicians are using retrograde aortic access for VT procedures, and researchers are increasingly investigating the technique. “Effective access is essential to supporting the growth of VT ablation,” said Fred P. Lampropoulos, Merit’s Chairman and Chief Executive Officer. “We are proud to take another significant step toward providing electrophysiologists with a valuable tool that helps fill this need in patient care.” ABOUT MERIT MEDICAL Founded in 1987, Merit Medical Systems, Inc. is engaged in the development, manufacture, and distribution of proprietary disposable medical devices used in interventional, diagnostic, and therapeutic procedures, particularly in cardiology, radiology, oncology, critical care, and endoscopy. Merit serves client hospitals worldwide with a domestic and international sales force and clinical support team totaling more than 700 individuals. Merit employs approximately 7,000 people worldwide. TRADEMARKS Unless noted otherwise, trademarks and registered trademarks used in this release are the property of Merit Medical Systems, Inc., its subsidiaries, or its licensors. CONTACTSPR/Media InquiriesSarah ComstockMerit Medical+1-801-432-2864 | sarah.comstock@merit.com INVESTOR INQUIRIESMike Piccinino, CFA, IRCWestwicke – ICR+1-443-213-0509 | mike.piccinino@westwicke.com Khurshid et al. 2018. “Frequency of Cardiac Rhythm Abnormalities in a Half Million Adults.” Circ Arrhythm Electrophysiol 11 (7): e006273. (PMID: 29954742)Adlan et al. 2019. “Retrograde Aortic Access During Ventricular Tachycardia Ablation: Indications, Techniques, and Challenges.” Journal of Cardiovascular Electrophysiology 30 (11): 2629–2639. (PMID: 31502368)