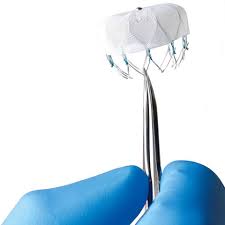
MARLBOROUGH, Mass., May 22, 2019 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) has initiated the OPTION trial to compare safety and effectiveness of the next-generation WATCHMAN FLX™ left atrial appendage closure (LAAC) platform to first-line oral anticoagulants (OAC) – including direct oral anticoagulants (DOAC) and warfarin – for stroke risk reduction in patients with […]
Rhythm
Results Announced from Two Renal Denervation Studies at EuroPCR Show Positive Outcomes in High-Risk Hypertensive Patients
DUBLIN and PARIS – May 22, 2019 – Investigators today unveiled late-breaking clinical data from a first-of-its-kind physician sponsored clinical trial. The data indicate that renal denervation (RDN) with the Medtronic Symplicity(TM) renal denervation system was associated with reduced occurrence of subclinical atrial fibrillation (AF) in a small subset of high-risk […]
Hemovent Receives CE Marking for MOBYBOX™ ECLS System
AACHEN, Germany–(BUSINESS WIRE)–Hemovent GmbH announced today that it has received CE Marking for its MOBYBOX™ ECLS System, allowing the company to market its innovative product throughout the European Union. MOBYBOX is a miniaturized heart/lung machine that uses Hemovent’s proprietary Bionique Flow Technologies as its platform. As the world’s first self-contained and fully integrated […]
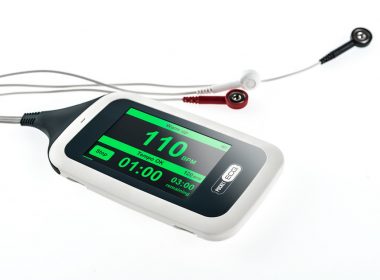
MediLynx Cardiac Monitoring Awarded Patent for Remote ECG Monitoring With Full Disclosure ECG Signal Transmission
PLANO, Texas, May 16, 2019 /PRNewswire/ — MediLynx Cardiac Monitoring LLC today announced the company has received a patent for its PocketECG full-disclosure ECG monitoring and transmission technology with patient symptomatic event reporting for remote cardiac monitoring. The patent covers use of the technology and methodology for arrhythmia monitoring and for remote or […]
FDA grants clearance for a heart murmur detection solution on a personal mobile device
eMurmur ID is an artificial intelligence-based solution that enables healthcare providers to detect and classify heart murmurs with expert-level accuracy OTTAWA, May 14, 2019 /PRNewswire/ – eMurmur® today announced that its flagship “eMurmur ID” solution has received FDA clearance, enabling a new era in heart murmur detection. eMurmur ID is a mobile and cloud […]
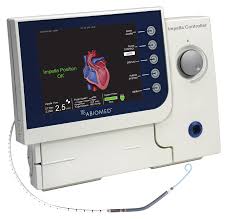
FDA Approves Impella 5.0 and Impella LD Extended Duration of Use to 14 Days for Cardiogenic Shock Derived from AMI or Cardiomyopathy
DANVERS, Mass.–(BUSINESS WIRE)–Abiomed (NASDAQ:ABMD) announces the U.S. FDA has approved the expansion of the Impella 5.0 and Impella LD PMA labeling for the treatment of cardiogenic shock. The expansion extends the duration of support for each pump from 6 days to 14 days. The Impella 5.0 and the Impella LD are forward flow heart pumps […]
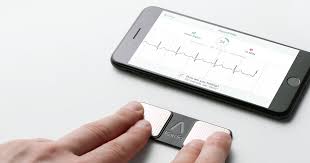
FDA Grants First Ever Clearance For Six-Lead Personal ECG Device
MOUNTAIN VIEW, Calif., May 13, 2019 /PRNewswire/ — AliveCor, the leader in FDA-cleared consumer electrocardiogram technology (ECG), today announced its third FDA clearance in three months, making KardiaMobile 6L the world’s first available six-lead personal ECG device. This highly anticipated clearance gives patients and their physicians an even more detailed view into patients’ […]
NEW STUDIES HIGHLIGHT UNDERUSE OF IMPLANTABLE CARDIAC DEVICES
DUBLIN and SAN FRANCISCO – May 10, 2019 – Three new studies show that patients who are medically indicated for implantable heart devices, including implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy (CRT), often do not receive needed therapy. The findings underscore gender and race treatment disparities, as well as a […]
CardioFocus Announces Impressive Results From Pivotal Confirmatory Study With The Breakthrough HeartLight® X3 System
MARLBOROUGH, Mass., May 10, 2019 /PRNewswire/ — CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for atrial fibrillation (AFib), today announced the presentation of results from its pivotal confirmatory study evaluating the HeartLight X3 System for the treatment of AFib. The results, which demonstrated superior procedural times and impressive procedural […]
Stereotaxis Announces Next Generation Robotic Magnetic Navigation System and Imaging System
Innovative robotic surgery system, Stereotaxis Genesis™ RMN, enriches robotic magnetic navigation with increased efficiency, reduced size, and enhanced lab experience Genesis RMN System to be available in combination with Stereotaxis Imaging Model S, a next-generation x-ray system offered as part of a complete robotic electrophysiology lab solution Combined technologies greatly […]