CARLSBAD, Calif., Jan. 22, 2019 /PRNewswire/ — Acutus Medical will highlight the benefits of its AcQMap advanced cardiac imaging and mapping visualization system at AF Symposium January 24-26 in Boston. The AcQMap system enables physicians to have a more precise approach to strategically address atrial arrhythmias by uncovering the true electrical activation pattern of the heart in […]
Rhythm
Abbott Offers a New Option for Physicians Treating Patients with Atrial Fibrillation
ABBOTT PARK, Ill., Jan. 21, 2019 /PRNewswire/ — Abbott (NYSE: ABT) today announced U.S. Food and Drug Administration (FDA) approval of the TactiCath™ Contact Force Ablation Catheter, Sensor Enabled™, a new ablation catheter designed to help physicians accurately and effectively treat atrial fibrillation (AFib). The approval further expands Abbott’s portfolio of cardiac ablation tools that integrate […]
Verily Study Watch Receives FDA 510(k) Clearance for ECG
In April of 2017, we launched Verily Study Watch, an investigational device for capturing health information from clinical research participants while serving as an easy-to-read watch for daily wear. Since then, Study Watch has been used by thousands of participants in clinical research studies run by Verily and through our partners, such […]
Johnson & Johnson Announces Research Study with Apple Watch to Help Improve AFib Outcomes Including Stroke Prevention
New Brunswick, NJ, January 17, 2019 — Johnson & Johnson (NYSE: JNJ) today announced that Janssen Pharmaceuticals, Inc., member of the Johnson & Johnson Family of Companies, entered into a research study in collaboration with Apple Inc. to investigate whether a new heart health program using an app from Johnson […]
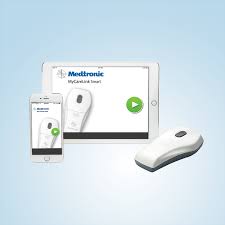
Medtronic Launches Mobile App That Communicates Directly with World’s First Smartphone-Connected Pacemakers
DUBLIN – January 15, 2019 – Medtronic plc (NYSE:MDT) today announced the launch of its MyCareLink Heart(TM) mobile app to support the world’s first and only portfolio of pacemakers that can communicate directly with patients’ smartphones and tablets. Compatible with Medtronic BlueSync(TM) technology-enabled pacemakers, the MyCareLink Heart mobile app is designed […]
New Heart Biometric Technology Confirms Your Identity and Measures Your Health via Advanced Auto Steering Wheel and Wearable Technology
LAS VEGAS–(BUSINESS WIRE)–EKG biometrics innovator B-Secur officially introduced its collection of powerful EKG authentication and wellness algorithms, HeartKey, at the 2019 Pepcom Digital Experience! This technology, with applications in the automotive and wearable technology industries, will soon be available in vehicles, top fitness trackers and smart wear of the future […]
CES 2019: Omron Healthcare Launches First Wearable Blood Pressure Monitor, Previews Breakthrough Monitor with EKG and New Digital Health Services
LAS VEGAS, Jan. 7, 2019 /PRNewswire/ — Omron Healthcare is advancing its mission of Going for Zero™ heart attacks and strokes with breakthrough new heart health technology offerings at the 2019 Consumer Electronics Show (CES). The global market leader of personal heart health and wellness technology is launching its highly-anticipated HeartGuide, the first […]
ARCA biopharma Updates Special Protocol Assessment Request to FDA for Gencaro Phase 3 Atrial Fibrillation Clinical Trial
WESTMINSTER, Colo., Dec. 20, 2018 (GLOBE NEWSWIRE) — ARCA biopharma, Inc. (Nasdaq: ABIO), a biopharmaceutical company applying a precision medicine approach to developing genetically-targeted therapies for cardiovascular diseases, today announced that it has submitted an amendment to its Special Protocol Assessment (SPA)request to the U.S. Food and Drug Administration (FDA). The amendment addresses […]
iRhythm Technologies to Present at the J.P. Morgan 37th Annual Healthcare Conference
SAN FRANCISCO, Dec. 19, 2018 (GLOBE NEWSWIRE) — iRhythm Technologies, Inc. (NASDAQ: IRTC), a leading digital health care solutions company focused on the advancement of cardiac care, today announced the company will be participating in the upcoming J.P. Morgan 37th Annual Healthcare Conference in San Francisco, CA. iRhythm’s management is scheduled to […]
HeartGuide, the highly-anticipated first wearable blood pressure monitor from Omron, available for pre-order on December 20th
LAKE FOREST, Ill., Dec. 20, 2018 /PRNewswire/ — Omron Healthcare, the number one doctor and pharmacist recommended blood pressure monitor brand, will open pre-orders for its new HeartGuide™, the first wearable blood pressure monitor, on December 20th at 12:00pm EST in the United States on OmronHealthcare.com. HeartGuide, an oscillometric blood pressure monitor in the design of a wrist watch, […]