IRVINE, Calif., Nov. 29, 2018 /PRNewswire/ — Johnson & Johnson Medical Devices Companies* announced today that Biosense Webster, Inc., a worldwide leader in the diagnosis and treatment of heart arrhythmias, has enrolled and treated the first patient in its STELLAR** U.S. Investigational Device Exemption (IDE) study. The study will evaluate the safety […]
Rhythm
CardioFocus Completes Enrollment In HeartLight® X3 Trial
MARLBOROUGH, Mass., Nov. 29, 2018 /PRNewswire/ — CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for atrial fibrillation (AFib), today announced completed enrollment in a trial to evaluate its next-generation HeartLight® X3 Endoscopic Ablation System. A total of 60 patients have been treated in this pivotal confirmatory trial with a one-month […]
Adagio Medical’s Persistent Atrial Fibrillation Cryoablation Study Demonstrates Early Single-Treatment Results Superior To Ablation Devices Requiring Two Procedures
LAGUNA HILLS, Calif., Nov. 28, 2018 /PRNewswire/ — Adagio Medical, Inc., developer of the iCLAS™ technology, the company’s ultra-low temperature intelligent continuous lesion ablation system for the treatment of cardiac arrhythmias, announces updates to its ongoing cryoablation study for Persistent Atrial Fibrillation (PsAF). The study is currently being conducted at two clinical sites […]
Innovative Health Receives FDA Clearances to Reprocess St. Jude Medical’s Advisor™ FL, Two Medtronic Diagnostic Catheters and Biosense Webster’s DECANAV®.
PHOENIX–(BUSINESS WIRE)–Innovative Health, a national leader in medical device reprocessing for the cardiology sector, announced it has received FDA clearances to reprocess the Advisor FL Circular Mapping Catheter, Medtronic’s Torqr™ and Marinr™, and Biosense Webster’s DECANAV. Three of these clearances were received within the last month alone. Innovative Health has already […]
New Harmonized Outcome Measures for Atrial Fibrillation Published in HeartRhythm
BOSTON, Nov. 15, 2018 /PRNewswire/ — OM1, a leading health outcomes and technology company, today announced the publication of new harmonized outcome measures for atrial fibrillation (AF) in HeartRhythm. OM1’s Richard Gliklichand Michelle Leavy collaborated on the development of the measures and co-authored the publication. The work was funded by the Agency for Healthcare Research and Quality (AHRQ) through an […]
Customer evaluation of CADScor®System compares favorably against existing methods
Interim Report, January – September 2018 Malmö, November 14, 2018 Customer evaluation of CADScor®System compares favorably against existing methods “During the quarter we proudly reported that Kristianstad Central Hospital in Sweden evaluated the CADScor®System with a positive outcome. Due to the good result the hospitals clinical management team has now […]
BioSig Technologies Announces New Research Program with Mayo Clinic
Santa Monica, CA, Nov. 13, 2018 (GLOBE NEWSWIRE) — BioSig Technologies, Inc. (NASDAQ: BSGM), a medical device company developing a proprietary biomedical signal processing platform designed to address an unmet technology need for the $4.6 billion electrophysiology (EP) marketplace, today announced that the Company entered into a new advanced research agreement with Mayo […]
GENETIC-AF Phase 2B Clinical Trial Atrial Fibrillation Burden (AFB) Results Presented at American Heart Association 2018 Scientific Sessions
WESTMINSTER, Colo., Nov. 12, 2018 (GLOBE NEWSWIRE) — ARCA biopharma, Inc. (Nasdaq: ABIO), a biopharmaceutical company applying a precision medicine approach to developing genetically-targeted therapies for cardiovascular diseases, today announced that data from the Atrial Fibrillation Burden (AFB) substudy of the Phase 2B GENETIC-AF clinical trial were presented November 11, 2018 in a poster session at […]
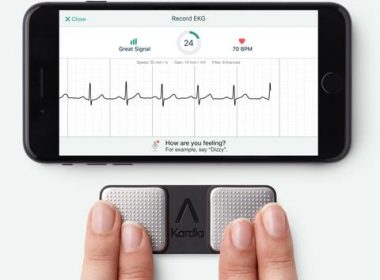
AliveCor’s Mobile ECG Technology Could Enable More Rapid Diagnosis and Treatment of Heart Attacks
MOUNTAIN VIEW, Calif., Nov. 12, 2018 /PRNewswire/ — AliveCor, the leader in FDA-cleared personal electrocardiogram (ECG) technology, has announced that a research version of its mobile technology can identify ST-elevation myocardial infarction (STEMI), the most severe form of heart attack. The findings of the ST LEUIS International Multicenter Study, to be announced at […]
Cardiva Medical Announces Positive Results of the AMBULATE Pivotal Study Evaluating the VASCADE MVP System Compared to Manual Compression for Multi-Vessel Closure Following Electrophysiology Procedures
SANTA CLARA, Calif. & CHICAGO–(BUSINESS WIRE)–Cardiva Medical, Inc., an innovator in the field of vascular closure, today announced that VASCADE® MVP, the first vascular closure system designed specifically for multi-access venous closure following electrophysiology procedures such as arrhythmia ablation, met its endpoints compared to manual compression in a pivotal clinical […]