NEW YORK and MELBOURNE, Australia, Oct. 02, 2018 (GLOBE NEWSWIRE) — Mesoblast Limited (ASX:MSB; Nasdaq:MESO) today announced that the 159-patient randomized placebo-controlled Phase 2b trial evaluating its allogeneic mesenchymal precursor cell (MPC) product candidate MPC-150-IM in the treatment of end-stage heart failure patients implanted with a left ventricular assist device (LVAD) has […]
Rhythm
CathVision Receives ISO 13485:2016 Certification
COPENHAGEN, Denmark, October 2, 2018 /PRNewswire/ — CathVision ApS, an early stage medical device company developing an advanced cardiac electrophysiology recording system, CUBE, announced today that the company received ISO 13485:2016 certification for Medical Device and Quality Management Systems from Lloyd’s Register. This ISO certification indicates that the company’s quality management system meets the […]
Adagio Medical Treats First Atrial Fibrillation Patients Using New One Shot+™ Cryoablation Catheter
LAGUNA HILLS, Calif., Oct. 1, 2018 /PRNewswire/ — Adagio Medical, Inc., the developer of the iCLAS™ technology, the company’s ultra-low temperature intelligent continuous lesion ablation system for the treatment of cardiac arrhythmias, announces that the first patients were successfully treated with its new One Shot+™ cryoablation catheter. The new catheter is aimed at […]
BIOSENSE WEBSTER ANNOUNCES FIRST PATIENTS ENROLLED IN POST-MARKET APPROVAL STUDY FOR ITS NOVEL TAG-INDEX GUIDED SOFTWARE
IRVINE, Calif., SEPTEMBER 26 – Johnson & Johnson Medical Devices Companies* announced today that Biosense Webster, Inc., a worldwide leader in the diagnosis and treatment of heart arrhythmias, has received approval from the U.S. Food and Drug Administration (FDA) for its VISITAG SURPOINT External Processing Unit and enrollment has begun […]
Bardy Diagnostics™ Selected as a Winner of the Children’s National Pediatric Medical Device Innovation Competition
SEATTLE, Sept. 26, 2018 /PRNewswire/ — Bardy Diagnostics, Inc., (“BardyDx”), a leading provider of ambulatory cardiac monitoring technologies and custom data solutions, including the Carnation Ambulatory Monitor (“CAM™”), the world’s first P-wave centric™ ambulatory cardiac monitor and arrhythmia detection device, announced it was selected as one of six winners of the […]
Huami Announces Launch of New Amazfit Health Band 1S and Introduces Groundbreaking Smart Wearable AI Chip, Huangshan-1
BEIJING, Sept. 18, 2018 /PRNewswire/ — Huami Corporation (“Huami” or the “Company”) (NYSE: HMI), a biometric and activity data-driven company with significant expertise in smart wearable technology, today announced the launch of the new generation health band, Amazfit Health Band 1S, which delivers new features and benefits powered by AI technologies and brings advanced […]
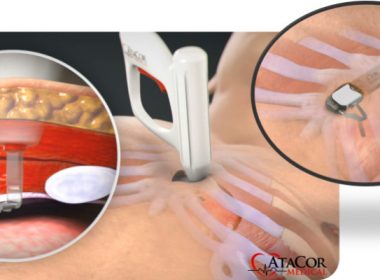
AtaCor Medical Raises $8.8M to Develop Substernal Cardiac Pacing System
SAN CLEMENTE, Calif.–(BUSINESS WIRE)–AtaCor Medical, Inc. (www.atacor.com) announced today that it has completed a $8.8M Series A financing. Co-led by Boston-based Broadview Ventures and Israel-based aMoon Ventures, the financing also includes participation from a corporate partner. The investment will support the continued development of AtaCor’s revolutionary extravascular substernal cardiac pacing […]
Apple Watch Series 4: Beautifully Redesigned With Breakthrough Communication, Fitness and Health Capabilities
CUPERTINO, Calif.–(BUSINESS WIRE)–Apple® today introduced Apple Watch® Series 4, redesigned and re-engineered to help users stay connected, be more active and manage their health in powerful new ways. While retaining the original iconic design, the fourth-generation Apple Watch has been refined, combining new hardware and software enhancements into a genuinely singular, […]
MEDTRONIC STATEMENT REGARDING RECENT FDA WARNING LETTERS
DUBLIN – September 11, 2018 – Today, Medtronic issued the following statement confirming it has received FDA warning letters (CMS #560736 and #562437) related to its Cardiac Rhythm and Heart Failure facilities located in Mounds View, Minn., and in Juncos, Puerto Rico. The warning letters reflect earlier FDA inspection reports from […]
With Latest FDA 510(k), physIQ Achieves Another Clearance as a Pioneer in AI Analytics
CHICAGO–(BUSINESS WIRE)–PhysIQ, a leader in applying AI analytics to biosensor data, today announced it has received FDA 510(k) clearance for its Atrial Fibrillation (AFib) detection analytics engine. The clearance extends physIQ’s portfolio of FDA 510(k) cleared analytics designed to generate clinical insight from wearable biosensors and further establishes physIQ as a […]