MOUNTAIN VIEW, Calif., Sept. 10, 201 /PRNewswire/ — AliveCor, recently recognized as the Most Innovative Company in Artificial Intelligence, today announced that the U.S. Food and Drug Administration has given the company’s KardiaK Software Platform the rarely granted designation of “Breakthrough Device.” This designation means that the FDA will consider the technology […]
Rhythm
InfoBionic Secures $50 Million in Financing to Support Continued Growth of the MoMe® Kardia System for Cardiac Arrhythmia Detection
BOSTON–(BUSINESS WIRE)–InfoBionic, a digital health company focused on patient-monitoring solutions for chronic disease management, today announced the completion of a $50 million financing to support continued commercial expansion of its MoMe® Kardia system for remote, wireless outpatient monitoring and diagnosis of cardiac arrhythmias. The financing round includes participation by Eagle Investments; Excel Venture Management; Safeguard Scientifics; Zaffre […]
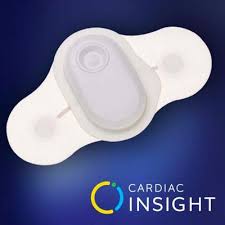
Cardiac Insight’s Wearable ECG Sensor, Cardea SOLO™, Continues to Attract Premier Cardiology Groups Across the U.S.; Announces Baltimore Heart Associates and Regional Cardiac Arrhythmia as Customers
KIRKLAND, Wash.–(BUSINESS WIRE)–Cardiac Insight, Inc., a leading U.S. developer of wearable biosensors and diagnostic software systems featuring proprietary automated data analysis algorithms, announced today that its wearable ECG Sensor, Cardea SOLO, continues to attract high-volume cardiology group practices across the U.S. The company unveiled Baltimore Heart Associates of Maryland (www.baltimoreheart.com) […]
Leviticus Cardio Announces Successful Completion of Third Chronic Animal Study
PETAH TIKVA, Israel, Sept. 6 2018 /PRNewswire/ — Leviticus Cardio, inventors of the versatile transcutaneous Coplanar Energy Transfer (CET) system for use with implanted left ventricular assist devices (LVADs), announces the successful completion of a 90-day preclinical chronic animal study to evaluate its CET technology in combination with a commercial heart pump. The […]
ULTRACONNECT from A&D Medical: The Power of Connected Health is Now Available
SAN JOSE, Calif., Sept. 5, 2018 /PRNewswire/ — A&D Medical, a global leader in connected health and biometric measurement devices and services, today announced its line of ULTRACONNECT blood pressure monitors is available nationwide, giving patients who are managing hypertension a new tool to better manage their heart health – with just a twist […]
Biotricity Developing ECG Patch for Anticipated Q1 2019 Release
REDWOOD CITY, Calif., Sept. 05, 2018 (GLOBE NEWSWIRE) — Biotricity Inc. (OTCQB: BTCY), a medical diagnostic and consumer healthcare technology company, today announced that it is developing “Biopatch,” an ECG patch which it hopes to release in Q1 2019. An extension of the company’s award-winning Bioflux device, Biopatch offers an alternative to the 3-lead system […]
HeartSciences Named Among ‘50 Smartest Companies of the Year 2018’ by The Silicon Review Magazine
SOUTHLAKE, Texas–(BUSINESS WIRE)–Silicon Review, a leading Business and Technology magazine has named HeartSciences, as one of the 50 Smartest Companies of the Year 2018. “The Silicon Review 50 Smartest Companies of the Year 2018 program identifies companies that mastered the discipline of smartness and stood high among the crowd,” said Sreshtha Banerjee, Editor-in-Chief […]
AtriCure Announces Completion of Patient Enrollment in the CONVERGE IDE Clinical Trial
MASON, Ohio–(BUSINESS WIRE)–AtriCure, Inc. (Nasdaq: ATRC), a leading innovator in surgical treatments for atrial fibrillation (Afib) and left atrial appendage management, today announced it has completed enrollment of the full cohort of 153 patients in the CONVERGE IDE clinical trial. The CONVERGE IDE trial is a landmark prospective, randomized trial […]
Daiichi Sankyo Presents First Snapshot Analyses from Global ETNA-AF Programme of Oral, Once-daily LIXIANA®▼ (edoxaban) in Patients with Nonvalvular Atrial Fibrillation in Routine Clinical Practice
MUNICH, Aug. 28, 2018 /PRNewswire/ — The Global ETNA-AF programme is a large and comprehensive single non-vitamin K oral anticoagulant (NOAC) repository of real-world data, as part of the Edoxaban Clinical Research Programme, EDOSURE Three snapshot analyses from baseline data add to growing evidence of the use of edoxaban in the real-world […]
Study Shows Improved Quality of Life and Reduced Symptoms In Patients Treated with Medtronic Cryoballoon
DUBLIN and MUNICH – August 28, 2018 – Medtronic plc (NYSE:MDT) today announced new findings from the CRYO4PERSISTENT AF clinical trial demonstrating improved quality of life, reduced symptoms from abnormal heart rhythms, and low incidence of reinterventions and repeat ablation procedures. The study evaluated patients with symptomatic persistent atrial fibrillation (AF) […]