Wireless connectivity enablement with AT&T helps Peerbridge expand access to AI-driven cardiac care LAS VEGAS, March 3, 2025 /PRNewswire/ — Peerbridge Health is ushering in the next generation of remote cardiac monitoring at the HIMSS Global Health Conference with the introduction of Cor…
Rhythm
Navi Medical Technologies’ Neonav® ECG Tip Location System Receives FDA 510(k) Clearance
Revolutionising Vascular Access Care for Critically Ill Newborns and Children Key Points: FDA 510(k) Clearance Neonav® ECG Tip Location System is now FDA-cleared, enabling its use in U.S. hospitals to enhance pediatric vascular access care. First-of-its-kind Innovation: Neonav® is the…
Volta Medical Artificial Intelligence-guided Cardiac Ablation Procedure Improves Treatment of Atrial Fibrillation
AI-Guided Cardiac Ablation in combination with pulmonary vein isolation was superior to the standard of care in the treatment of Persistent Atrial FibrillationVolta Medical Announces Publication of Landmark Clinical Trial in Nature Medicine. This trial marks the first large-scale demonstration of the benefits of AI in interventional cardiology. MARSEILLE, France, Feb. 14, 2025 (GLOBE NEWSWIRE) — Volta Medical, a pioneering health technology company developing artificial intelligence (AI) solutions to assist electrophysiologists, today announced the publication of the landmark TAILORED-AF clinical trial in Nature Medicine, which demonstrated that an AI-guided procedure for persistent atrial fibrillation (AF) in combination with conventional pulmonary vein isolation (PVI) treatment, resulted in better outcomes than PVI alone. In particular, the study showed superiority in the percentage of patients that achieved freedom from atrial fibrillation (AF) with or without anti-arrhythmic drugs at 12 months after a tailored cardiac ablation guided by AI in combination with PVI when compared to PVI alone. Despite advances in catheter ablation technology, persistent AF remains one of the most challenging subtypes of atrial fibrillation to treat, affecting over 70% of all patients with atrial fibrillation globally.i The TAILORED-AF clinical trial is the first large-scale transatlantic randomized controlled trial (RCT) of ablation in a persistent atrial fibrillation (AF) population to show the benefit of going beyond conventional pulmonary vein isolation (PVI)-only extra-pulmonary vein procedure. Previous studies looking at ablation strategies for persistent AF patients have not demonstrated superior efficacy to PVI alone. The Volta AF-Xplorer™ is a digital AI companion designed to assist cardiologists with real-time identification of specific abnormal electrograms (EGMs) known as spatio-temporal dispersed EGMs, this technology was used in the TAILORED-AF trial. “Previously, there has not been a replicable, effective treatment strategy for patients with persistent atrial fibrillation. Volta’s AI solution finally offers a solution for this large and underserved patient population,” said Théophile Mohr-Durdez, CEO and co-founder of Volta Medical. “The TAILORED-AF trial, highlights AI’s ability to help physicians treat cardiovascular disease and improve patient outcomes. In fact, this is the first large-scale international RCT in interventional cardiology demonstrating superior efficacy through the use of AI.” Summary and Results: Tailored vs. Anatomical Ablation Strategy for Persistent Atrial Fibrillation (TAILORED-AF) Study In the clinical trial, adults with symptomatic persistent or long-standing persistent AF who were candidates for a first-time ablation were enrolled in Europe and the United States. A total of 187 patients underwent a tailored cardiac ablation guided by Volta’s AI in addition to PVI (Tailored cohort), and 183 patients received the conventional treatment of PVI-only (Anatomical cohort) and all were followed up for 12 months. A total of 51 electrophysiologists at 26 centers in 5 countries across the US and the EU participated. For access to the full publication visit https://www.nature.com/articles/s41591-025-03517-w. The trial met the primary endpoint by demonstrating superior results in patients assigned to the Tailored cohort compared to the Anatomical cohort. 88% of patients in the Tailored cohort experienced freedom from AF 12 months after one procedure with or without anti-arrhythmic drugs compared to 70% in the Anatomical cohort (log rank p < 0.0001).In the Tailored cohort, patients experienced a higher rate of freedom from any arrhythmia after 1.2 procedures than in the Anatomical cohort (79% vs. 71%, log rank p < 0.01). Recurrences in the Tailored cohort were regarded as a “simplification” of AF which are generally easier to ablate and can be seen as a step towards stable sinus rhythm.The trial also examined several pre-specified secondary endpoints and a pre-specified subgroup analysis of patients with sustained persistent AF lasting 6 months or longer prior to enrollment. These patients represent a more advanced AF disease progression. Patients in the Tailored cohort experienced a significantly higher rate of freedom from any arrhythmia after one single procedure than in the Anatomical cohort (62% vs. 48%, log rank p = 0.04).The safety endpoint did not differ between the groups, although the procedure and ablation time were twice as long in the Tailored arm, consistent with treatment time for other PVI+ methods. “Atrial fibrillation, when left untreated, doubles the risk of heart-related deaths and is associated with a 5-fold increased risk for stroke. However, advancements in AI are transforming this landscape,” said Seth Goldbarg, MD, FACC, FHRS, Cardiologist, NewYork-Presbyterian, Queens. “This trial featuring AI-guided assessment of AF represents a true milestone for improvement of outcomes for this underserved and difficult to treat patient community.” About Atrial Fibrillation The American Heart Association defines AF as a quivering or irregular heartbeat (arrhythmia) that can lead to blood clots, stroke, heart failure, and other heart-related complications.ii Approximately 33 million patients worldwide are living with AF.iii,iv Even though untreated AF doubles the risk of heart-related deaths and is associated with a 5-fold increased risk for stroke, many patients are unaware that AF is a serious condition. It is estimated that only 15% of the eligible 2.5 million US patients with AF are currently being treated with catheter ablation.v About Volta Medical Volta Medical is a health technology company developing artificial intelligence software solutions with the aim of assisting cardiac electrophysiologists during arrhythmia treatment procedures to improve clinical outcomes for patients. Founded by three physicians and a data scientist in 2016 in Marseille, France, the company’s mission is to improve cardiac arrhythmia management by developing state-of-the-art, data-driven medical devices trained on large databases of procedural data. The Volta AF-Xplorer™ is a digital AI companion designed to assist cardiologists with real-time identification of specific abnormal electrograms (EGMs) known as spatio-temporal dispersed EGMs during AF and atrial tachycardia procedures. The AF-Xplorer™ has been engineered for versatility and its use has been demonstrated with the most popular AF mapping and recording systems, as well as with the most common ablation modalities. The solution is U.S. FDA 510(k) cleared and European CE Mark approved. For more information, visit the company’s website at www.volta-medical.com. Volta Medical has created a therapy awareness program AI for Persistent AF Care designed to educate the underserved AF patient community, for more information, visit www.aiforafib.com. U.S. Media ContactGlenn SilverFinn Partnersglenn.silver@finnpartners.comVolta ContactJeff Martin, SVP of Global MarketingVolta Medical jeffrey.martin@volta-medical.com References David DeLurgio, Jaswinder Gill, Syed Ahsan, Riyaz Kaba, Kristen M Plasseraud, Michael E Halkos, Hybrid Convergent Procedure for the Treatment of Persistent and Long-standing Persistent Atrial Fibrillation, Arrhythmia & Electrophysiology Review 2021;10(3):198–204. https://doi.org/10.15420/aer.2021.24https://www.heart.org/en/health-topics/atrial-fibrillation [last accessed June 9, 2023]Colilla S, Crow A, Petku W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol 2013; 112:1142–1147. DOI: 10.1016/j.amjcard.2013.05.063https://kompetenznetz-vorhofflimmern.de/en [last accessed June 9, 2023]https://www.mddionline.com/cardiovascular/medtronic-makes-a-double-play-for-atrial-fibrillation
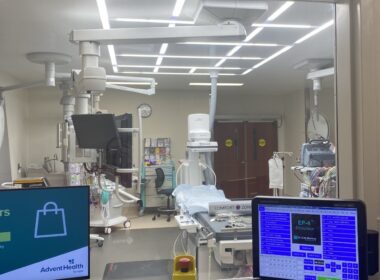
Pepin Heart Institute at AdventHealth Tampa expands with fourth electrophysiology lab
TAMPA, Fla., Feb. 10, 2025 /PRNewswire/ — The AdventHealth Pepin Heart Institute has further strengthened its commitment to providing world-class cardiac care by opening its fourth dedicated Electrophysiology (EP) Lab. This state-of-the-art expansion will allow for even more advanced…
SMH #1 in World for “Watchman” Treatment
Innovative device prevents strokes – without medication – in at-risk heart patients SARASOTA, Fla., Feb. 6, 2025 /PRNewswire/ — Sarasota Memorial Hospital has earned the #1 spot among the world’s WATCHMAN providers protecting people with atrial fibrillation. This week, the Sarasota…
Acarix Partners with Geo-Med to Extend the Availability of the CADScor System for Veterans
OKLAHOMA CITY, Feb. 5, 2025 /PRNewswire/ — Acarix, a pioneer in the development of groundbreaking acoustic-based cardiac diagnostic solutions, is proud to announce a new strategic partnership with Geo-Med, LLC, a renowned Service-Disabled Veteran-Owned Small Business that specializes in…
Stereotaxis Receives CE Mark Approval for the MAGiC Ablation Catheter
ST. LOUIS, Jan. 27, 2025 (GLOBE NEWSWIRE) — Stereotaxis (NYSE: STXS), a pioneer and global leader in surgical robotics for minimally invasive endovascular intervention, today announced that it received European CE Mark approval for the MAGiC™ ablation catheter. This approval is a significant milestone for Stereotaxis and for the community of physicians pioneering robotics in electrophysiology. It is reflective of the company’s commitment to advancing significant innovations that make robotics increasingly impactful across interventional medicine.
Data Published Today in the New England Journal of Medicine Demonstrates Anthos Therapeutics’ novel Factor XI inhibitor, Abelacimab 150mg, Reduced Major or Clinically Relevant Non-Major Bleeding by 62% Compared to Rivaroxaban (Xarelto) in Patients with Atrial Fibrillation
Secondary endpoints also showed a highly significant 67% reduction in major bleeding and an 89% reduction in gastrointestinal (GI) bleeding The company’s AZALEA-TIMI 71 study is the longest head-to-head study of a Factor XI inhibitor vs. a direct oral anticoagulant (DOAC) CAMBRIDGE, Mass., Jan. 22, 2025 (GLOBE NEWSWIRE) — Anthos Therapeutics, Inc., a transformative, clinical-stage…
A New Scientific Approach Converting Atrial Fibrillation With Oral Magnesium Supplements
BOZEMAN, Mont., Jan. 22, 2025 /PRNewswire/ — Atrial fibrillation (AF) is the most common abnormal heart rhythm affecting 6 million people in the USA and can cause strokes and heart failure. Conventional treatments are successful but may need to be repeated, have inherent risks, and are…
Pulse Biosciences Announces Late-Breaking Data from its Nanosecond PFA 360° Cardiac Catheter System First-In-Human Feasibility Study Presented at the AF Symposium
MIAMI–(BUSINESS WIRE)–Pulse Biosciences, Inc. (Nasdaq: PLSE), a company leveraging its novel and proprietary Nanosecond Pulsed Field Ablation™ (nsPFA™) technology, today announced the late-breaking data from its Nanosecond PFA 360° Cardiac Catheter System first-in-human feasibility study, which data were recently presented at the 30th Annual AF Symposium 2025 meeting. “This novel technology […]