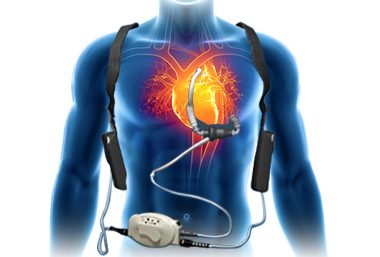
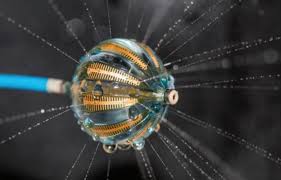
LAKE OSWEGO, Ore. and SILVER SPRING, Md., April 3, 2018 /PRNewswire/ — BIOTRONIK US and Aziyo today announced a strategic agreement allowing BIOTRONIK to distribute Aziyo’s CanGaroo® extracellular matrix (ECM) cardiovascular implantable electronic device (CIED) envelopes in the United States. BioEnvelope will be available from BIOTRONIK starting in April 2018. BioEnvelope reduces scar formation and has been shown to mitigate the […]
Rhythm
CorWave is Set to Receive about $3.5 Million in Financing Through France’s Worldwide Innovation Challenge for Its NovaPulse Program, a Mini-Invasive Solution for Cardiac Support
CLICHY, France–(BUSINESS WIRE) CorWave, a French medical technology company that develops innovative implanted cardiac support devices for patients suffering from heart failure, announced today that it has been awarded almost $3.5 million in financing for its R&D program NovaPulse in the Silver Economy category. This is part of phase 2 of […]
Bardy Diagnostics™ Names Ken Nelson Chief Commercial Officer
SEATTLE, April 2, 2018 /PRNewswire/ — Bardy Diagnostics, Inc., (“BardyDx”), a leading provider of ambulatory cardiac monitoring technologies and custom data solutions, including the Carnation Ambulatory Monitor (“CAM™”), the world’s smallest and lightest P-wave centric™ ambulatory cardiac monitor and arrhythmia detection device, announced today the appointment of Kenneth W. Nelson III as Chief Commercial Officer […]
EP Solutions’ Scientific and Mathematics Advisory Boards Endorse Company Strategy
YVERDON-LES-BAINS, Switzerland, March 29, 2018 /PRNewswire/ — Dr. Joaquin Azpilicueta, M.D., EP Solutions CEO reports the outcome of the meetings with the Scientific Advisory Board and the Mathematics Advisory Board celebrated in February and March 2018 respectively. The Scientific Advisory Board, with members that hold prominent positions in the cardiac electrophysiology community like Prof. Dr. Claudio Tondo, […]
Abbott Initiates Trial to Evaluate Improved Survival And Outcomes with the CardioMEMS Monitor
ABBOTT PARK, Ill., March 29, 2018 /PRNewswire/ — Abbott (NYSE: ABT) announced today the company has initiated the landmark GUIDE-HF clinical trial using the CardioMEMS™ HF System. The GUIDE-HF trial will study whether the CardioMEMS device can improve survival and quality of life for people living with New York Heart Association (NYHA) Class II – IV heart failure. The […]
Late-breaking Clinical Trial Data Further Demonstrate Safety and Effectiveness of the RHYTHMIA™ Mapping System
BARCELONA, Spain and MARLBOROUGH, Mass., March 20, 2018 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) announced real-world data from the TRUE-HD study during a late-breaking clinical trial session today at the annual congress of the European Heart Rhythm Association (EHRA) in Barcelona, Spain. The data demonstrated the RHYTHMIA™ Mapping System, when paired with the INTELLAMAP […]
BIOSENSE WEBSTER, INC. ANNOUNCES FIRST PATIENT TREATED IN STUDY OF MULTI-ELECTRODE RADIOFREQUENCY BALLOON CATHETER
Multicenter Study to Evaluate Balloon Ablation Catheter for Safety and Effectiveness in Achieving Pulmonary Vein Isolation in Treatment of Paroxysmal Atrial Fibrillation IRVINE, CA, MARCH 15 – Johnson & Johnson Medical Devices Companies* announced today that Biosense Webster, Inc., a worldwide leader in the diagnosis and treatment of heart arrhythmias, […]
Texas Cardiac Arrhythmia Institute at St. David’s Medical Center draws cardiac experts from across the globe for EPLive 2018
AUSTIN, Texas, March 19, 2018 /PRNewswire/ — On March 1 and 2, 2018, the Texas Cardiac Arrhythmia Institute (TCAI) at St. David’s Medical Center hosted its fourth international symposium on complex arrhythmias, EPLive 2018. Nearly 200 leaders in the field of electrophysiology (EP) from Europe, Asia and Latin America, as […]
Global Digital Blood Pressure Monitors Market 2018-2022 – Key Vendors are A&D, Microlife, Welch Allyn, Omron Healthcare & Smiths Medical
DUBLIN, March 14, 2018 /PRNewswire/ — The “Global Digital Blood Pressure Monitors Market 2018-2022” report has been added to ResearchAndMarkets.com’s offering. The global digital blood pressure monitors market to grow at a CAGR of 9.79% during the period 2018-2022. Global Digital Blood Pressure Monitors Market 2018-2022, has been prepared based on an in-depth market analysis with […]
PATIENT ENROLLMENT COMPLETED IN U.S. IDE STUDY OF THERMOCOOL SMARTTOUCH® SF CATHETER FOR TREATMENT OF PERSISTENT ATRIAL FIBRILLATION
IRVINE, CA, — March 13, 2018 — Johnson & Johnson Medical Devices Companies* announced today that Biosense Webster, Inc., a worldwide leader in the diagnosis and treatment of heart arrhythmias, has completed patient enrollment in its U.S. Investigational Device Exemption (IDE) study of the THERMOCOOL SMARTTOUCH® SF Catheter. The prospective, […]