ORLANDO, Fla., March 12, 2018 /PRNewswire/ — AliveCor, the leader in FDA-cleared personal electrocardiogram (ECG) technology, today announced findings from two new studies that further support the use of its portable ECG devices to provide groundbreaking health monitoring capabilities. A new Cleveland Clinic study — which marks the first time Apple […]
Rhythm
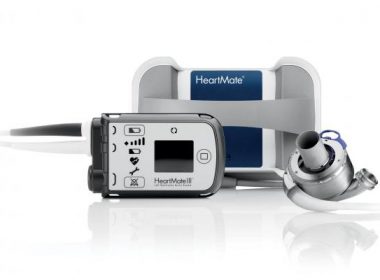
New Long-Term Data Show Improved Survival and Lower Rates of Stroke and Pump Thrombosis for Abbott’s HeartMate 3 Heart Pump
ORLANDO, Fla., March 11, 2018 /PRNewswire/ — Abbott (NYSE: ABT) announced new late-breaking clinical trial data from the MOMENTUM 3 clinical study, the largest left ventricular assist device (LVAD) trial in the world to evaluate patients in need of both short-term and long-term support in a single study. The data were published online […]
New Late-Breaking Study Finds Wearable Electrocardiogram (ECG) Monitoring Patch Can Detect Atrial Fibrillation Earlier and More Efficiently than Routine Care
ORLANDO, Fla., March 10, 2018 /PRNewswire/ — The Janssen Pharmaceutical Companies of Johnson & Johnson today announced late-breaking results of a new home-based clinical trial showing that a wearable continuous electrocardiogram (ECG) monitoring patch can identify people with asymptomatic atrial fibrillation (AFib) earlier and more efficiently than routine care. One-year findings from […]
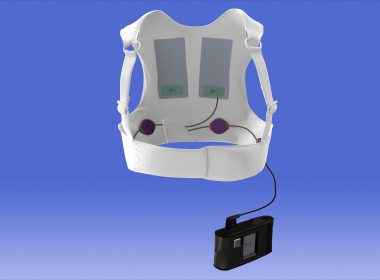
LifeVest Wearable Defibrillator Reduces Total Mortality By 36 Percent At 90 Days
CHELMSFORD, Mass., March 10, 2018 /PRNewswire/ — ZOLL® Medical Corporation, an Asahi Kasei Group Company that manufactures medical devices and related software solutions, announced today the results from the “Vest Prevention of Early Sudden Death Trial (VEST).” The study demonstrated that use of the LifeVest® wearable cardioverter defibrillator(WCD) reduced total mortality by 36 percent in the first […]
Boston Scientific Announces Scheduled Presentations at EHRA 2018 Congress
MARLBOROUGH, Mass., March 12, 2018 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) today announced key data presentations that will be featured at the annual congress of the European Heart Rhythm Association (EHRA) in Barcelona, Spain, on March 18-20. Notably, there will be three late-breaking clinical trials which include the following: Outcomes from TRUE-HD, a large registry […]
LivaNova Enters into Definitive Agreement to Divest Its Cardiac Rhythm Management Business Franchise to MicroPort Scientific Corporation
LONDON–(BUSINESS WIRE)– LivaNova PLC (NASDAQ:LIVN) (“LivaNova”) today announced that it has entered into a definitive Stock and Asset Purchase Agreement (“Purchase Agreement”) to divest its Cardiac Rhythm Management business franchise to MicroPort Scientific Corporation (“MicroPort”). LivaNova and MicroPort executed and delivered the Purchase Agreement, pursuant to the terms of a binding Letter of Intent, […]
EBR Systems, Inc. Initiates Global Trial of World’s Only Wireless CRT Pacing Technology
SUNNYVALE, Calif.–(BUSINESS WIRE)–EBR Systems, Inc., developer of the world’s only wireless cardiac pacing system for heart failure, today announced enrollment of the first patients in the global SOLVE-CRT (Stimulation of the Left Ventricular Endocardium for Cardiac Resynchronization Therapy) clinical trial. The first patients were enrolled by Prof. Dr. Christian Butter […]
Cardiac Insight, Inc. to Showcase Wearable Devices and Systems for Atrial Fibrillation and Sudden Cardiac Death Risk Detection at the American College of Cardiology’s 67th Annual Scientific Session, March 10-12, Booth #2417
KIRKLAND, Wash.–(BUSINESS WIRE)–Cardiac Insight, Inc., a leading U.S. developer of wearable medical devices and diagnostic software, announced today the company will showcase its one-of-a-kind cardiac devices and software systems, featuring proprietary algorithms, for the first time at a major cardiology exposition at the annual American College of Cardiology’s (ACC) 67th Annual […]
Nihon Kohden Launches Aireeg® WEE-1200 Wireless EEG System at HIMSS18
IRVINE, Calif.–(BUSINESS WIRE)– Nihon Kohden Corporation, a U.S. market leader in precision medical products and services, has launched its aireeg®WEE-1200 wireless electroencephalogram (EEG) system designed specifically for patient comfort and easy access to real-time patient data during long-term epilepsy monitoring, intensive care, and routine EEG environments. The smart, safe and secure […]
Safety and Effectiveness of BioTrace Medical’s Tempo® Lead Further Demonstrated in Multi-Center Analysis of More Than 200 Cardiac Procedures
MENLO PARK, Calif. & ORLANDO, Fla.–(BUSINESS WIRE)–BioTrace Medical, Inc., the leader in innovative temporary pacing technology, today announced positive safety and effectiveness data from a physician-sponsored U.S. study of the company’s Tempo® Temporary Pacing Lead. Results from a multi-center retrospective analysis of 224 U.S. interventional cardiac procedures, including transcatheter aortic valve […]