KIRKLAND, Wash.–(BUSINESS WIRE)– Cardiac Insight, a U.S. developer of wearable medical devices and diagnostic software, announced today that the company has secured its first cardiology customers for its wearable ECG sensor device, Cardea SOLO, in Texas and several other states. Early physician and patient experiences with Cardea SOLO are confirming the […]
Rhythm
EBR Systems, Inc. Raises $50 Million to Complete Global Heart Failure Trial
SUNNYVALE, Calif.–(BUSINESS WIRE)–EBR Systems, Inc., developer of the world’s first and only wireless cardiac pacing system for heart failure, has raised $50 million to conduct the global SOLVE-CRT study. The financing was led by Australian private equity firms M.H. Carnegie & Co. and Brandon Capital Partners and included participation by […]
BioSig Technologies Adds Role of Chief Regulatory and Compliance Officer in Preparation for FDA Submission
Santa Monica, CA, Nov. 14, 2017 (GLOBE NEWSWIRE) — BioSig Technologies(OTCQB: BSGM), a medical device company developing a proprietary biomedical signal processing platform designed to address an unmet technology need for the $4.6 billion electrophysiology (EP) marketplace, today announced that the Company has engaged Tiffini Wittwer as Chief Regulatory and Compliance Officer […]
Aziyo Launches CanGaroo Bio Envelope for Subcutaneous Implantable Cardioverter-Defibrillators
SILVER SPRING, Md.–(BUSINESS WIRE)– Aziyo Biologics, a fully integrated regenerative medicine company, today announced the launch of a new CanGaroo Bio Envelope specifically for use with subcutaneous implantable cardioverter-defibrillators (S-ICDs). This is the only cardiac implantable electronic device (CIED) envelope available for use with S-ICDs. CanGaroo is a natural extracellular matrix (ECM) scaffold that […]
Five-year Follow-up Data Demonstrate the WATCHMAN™ Left Atrial Appendage Closure Device Provides Stroke Risk Reduction Comparable to Warfarin Therapy
DENVER and MARLBOROUGH, Mass., Nov. 2, 2017 /PRNewswire/ — Boston Scientific (NYSE: BSX) announced final five-year outcomes data from the PREVAIL study of the WATCHMAN™ Left Atrial Appendage Closure (LAAC) Device today during a late-breaking clinical trial session at the 29th Transcatheter Cardiovascular Therapeutics (TCT), the annual scientific symposium of the Cardiovascular Research Foundation, in Denver. The […]
Imricor and MiRTLE Medical Announce Joint Development Agreement to Deliver 12-Lead ECG for Real-Time MRI-Guided Cardiac Ablations
MINNEAPOLIS–(BUSINESS WIRE)–Imricor and MiRTLE Medical announce a joint development agreement to fully integrate MiRTLE’s MR compatible 12-lead ECG system with Imricor’s Advantage-MR™ EP Recorder/Stimulator System. This integration, along with Imricor’s MR compatible catheters, will allow physicians to perform complex cardiac ablations, such as for treating ventricular tachycardia and atrial fibrillation, under real-time MRI-guidance. […]
BioTrace Medical Announces Key Events Featuring Tempo® Temporary Pacing Lead at TCT 2017
SAN CARLOS, Calif.–(BUSINESS WIRE)–BioTrace Medical, Inc., today announced the company’s key activities at the Transcatheter Cardiovascular Therapeutics (TCT) 2017 meeting, which will take place October 29 – November 2 at the Colorado Convention Center in Denver. The company’s Tempo® Temporary Pacing Lead will be featured in a breakfast symposium titled: “The […]
SentreHEART Announces Participation at Transcatheter Cardiovascular Therapeutics 2017
REDWOOD CITY, Calif.–(BUSINESS WIRE)–SentreHEART, Inc., the manufacturer of the LARIAT® Suture Delivery Device (LARIAT) will be participating in the Transcatheter Cardiovasular Therapeutics (TCT), the annual scientific symposium of the Cardiovascular Research Foundation, in Denver, CO October 29 – November 2, 2017. The symposium’s agenda will include several sessions dedicated to […]
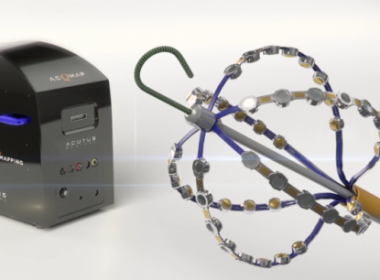
Acutus Medical® Receives FDA Clearance for Advanced Cardiac Mapping Technology for Complex Arrhythmias
CARLSBAD, Calif., Oct. 24, 2017 /PRNewswire/ — Acutus Medical® today announced that the U.S. Food and Drug Administration has cleared the AcQMap® High Resolution Imaging and Mapping System and the AcQMap® 3D Imaging and Mapping Catheter for use in patients for whom electrophysiology procedures have been prescribed. The Company plans to introduce initial commercial systems […]
BioSig Technologies Adds Role of Manufacturing Project Leader as Company Continues to Advance Towards Commercialization
Minneapolis, MN, Oct. 24, 2017 (GLOBE NEWSWIRE) — BioSig Technologies(OTCQB: BSGM), a medical device company developing a proprietary platform designed to address an unmet technology need for the $4.6 billion electrophysiology (EP) marketplace, today announced that the Company has engaged Quintain Project Solutions LLC as the manufacturing project management leader for […]