CHELMSFORD, Mass.–(BUSINESS WIRE)–ZOLL®, an Asahi Kasei company that manufactures medical devices and related software solutions, announced today that the first patient has been enrolled in the SuperSaturated oxygen Comprehensive Observational REgistry (SSCORE). The prospective study is designed to provide further evidence of the efficacy of SuperSaturated Oxygen (SSO2) Therapy to reduce heart failure and […]
Rhythm
Funding Supports Neuranics’ Groundbreaking AI Technology for Remote Heart Health Monitoring
September 20, 2024 10:27 AM Eastern Daylight Time GLASGOW, Scotland–(BUSINESS WIRE)–Maja Schmidt, in collaboration with Neuranics Limited and the University of Edinburgh, has been awarded the highly coveted Royal Commission for the Exhibition of 1851 Industrial Fellowship for her innovative AI-driven remote heart health monitoring project. This initiative aims to […]
Elutia Announces New Peer Reviewed Publication Highlighting the Robustness of EluPro™, Company’s Antibiotic-Eluting BioEnvelope for Implantable Devices
EluPro eradicated bacteria commonly associated with cardiac implant-related infections in an established preclinical infection model
AFIB: A SILENT CAUSE OF HEART FAILURE AND STROKE
*** September is National Atrial Fibrillation Awareness Month *** SAN FRANCISCO, Sept. 17, 2024 /PRNewswire/ — BACKGROUND: We all know someone who’s had a heart scare – an unusual flutter or disconcerting palpitations. The potential cause? A little-known and often silent heart health…
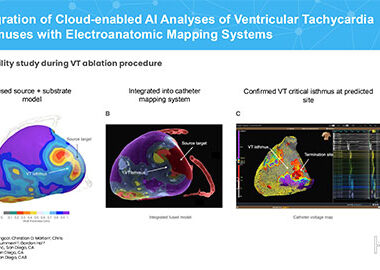
Vektor Medical Unveils Groundbreaking Research on AI and Deep Learning Innovations in Ventricular Tachycardia Analysis
September 06, 2024 07:59 AM Eastern Daylight Time ATLANTA–(BUSINESS WIRE)–Vektor Medical, a leader in non-invasive, AI-powered arrhythmia analysis technology, will present findings today at HRX 2024. The two studies showcase how Vektor’s advanced technologies uniquely leverage AI and deep learning to assess wall thickness and scar tissue in ventricular tachycardia […]
Medtronic EV ICD global pivotal trial final results demonstrate high ATP success and effective defibrillation: ESC Congress 2024
Benefits of transvenous implantable defibrillators, including anti-tachycardia pacing (ATP), in a single device implanted safely outside the vascular space: 77% ATP success rate, in line with transvenous ICDs Avoided shocks in nearly half of all ventricular tachycardia/ventricular fibrillation (VT/VF) episodes through 30.6 months average follow-up LONDON and GALWAY, Sept. […]
Elutia Announces First Patient Implant of EluPro™, the World’s First Drug-Eluting BioEnvelope for Cardiac Pacemakers and Neurostimulators
SILVER SPRING, Md., Sept. 05, 2024 (GLOBE NEWSWIRE) — Elutia Inc. (Nasdaq: ELUT) (“Elutia”), a pioneer in drug-eluting biomatrix products, today announced a landmark achievement with the first-ever patient implant of EluPro®, the world’s first antibiotic-eluting biologic envelope cleared by the U.S. Food and Drug Administration (FDA). The groundbreaking procedure was performed by John Catanzaro, MD, MBA, Chief, Division of Cardiology, Director, Cardiology Services, and Program Director of Clinical Cardiac Electrophysiology Fellowship at East Carolina University Health Medical Center in Greenville, North Carolina.
Stereotaxis Robotic Technology to be Featured during Heart Rhythm Society’s HRX Congress
ST. LOUIS, Sept. 03, 2024 (GLOBE NEWSWIRE) — Stereotaxis (NYSE: STXS), a pioneer and global leader in surgical robotics for minimally invasive endovascular intervention, today announced that its technology will be prominently featured during the upcoming HRX digital health conference taking place September 5-7, 2024, in Atlanta, Georgia.
DRAI MARTINI: Artificial Intelligence Beats Human Readers at Arrhythmia Detection
WARSAW, Poland, Sept. 2, 2024 /PRNewswire/ — New research presented at the ESC Congress 2024 reveals that a novel artificial intelligence (AI) algorithm has significantly higher sensitivity than human specialists in detecting heart rhythm disorders on long ECG recordings. The DRAI…
BioCardia Announces FDA Market Clearance of Morph® DNA™ Steerable Introducer Product Family
SUNNYVALE, Calif., Aug. 29, 2024 (GLOBE NEWSWIRE) — BioCardia, Inc. [Nasdaq: BCDA], a global leader in cellular and cell-derived therapeutics for the treatment of cardiovascular and pulmonary diseases, announced today that the Food and Drug Administration (FDA) has cleared BioCardia to market the Morph DNA Steerable Introducer product family, subject to the general controls provisions of the Federal Food, Drug, and Cosmetic Act.