SAN FRANCISCO–(BUSINESS WIRE)–Element Science, an innovative health technology company pioneering a digital wearable platform for high-risk cardiovascular patients, announced that it has received the European Union’s CE mark certification and Great Britain’s UK Conformity Assessed (UKCA) marking for its novel Patch Wearable Cardioverter Defibrillator (P-WCD) from its Notified Body, the […]
Rhythm
ABBOTT ANNOUNCES FIRST GLOBAL PROCEDURES IN A CLINICAL TRIAL OF ITS VOLT™ PULSED FIELD ABLATION SYSTEM TO TREAT PATIENTS WITH ABNORMAL HEART RHYTHMS
ABBOTT PARK, Ill., Jan. 18, 2024 /PRNewswire/ — Abbott (NYSE: ABT) today announced the first global procedures have been conducted using the company’s new Volt™ Pulsed Field Ablation (PFA) System to treat patients battling common abnormal heart rhythms such as atrial fibrillation (AFib). Over 30 patients were recently treated in Australia as part of […]
PulseAI’s Groundbreaking AI Algorithm Enhances Consumer ECG Diagnostics
Innovative AI Application Improves Cardiac Monitoring and Diagnostic Accuracy BELFAST, Northern Ireland , Jan. 11, 2024 /PRNewswire/ — PulseAI, a leader in artificial intelligence healthcare solutions, today announced a significant advancement in consumer device electrocardiogram (ECG)…
Biosense Webster Announces Regulatory Approval of VARIPULSE™ Pulsed Field Ablation (PFA) Platform in Japan
First regulatory approval for the first-of-its-kind, fully-integrated PFA system with a simple and reproducible workflow Integrated with the world’s leading CARTO™ 3D cardiac mapping system for the treatment of symptomatic drug refractory recurrent paroxysmal atrial fibrillation (AFib)…
Stereotaxis Announces First Patients Successfully Treated Using MAGiC Ablation Catheter
ST. LOUIS, Jan. 08, 2024 (GLOBE NEWSWIRE) — Stereotaxis (NYSE: STXS), a pioneer in surgical robotics for minimally invasive endovascular intervention, today announced that the first patients have been successfully treated using its Magnetic Interventional Ablation Catheter, MAGiC™. Stereotaxis’ MAGiC catheter is a robotically navigated magnetic ablation catheter designed to […]
Windtree Therapeutics Announces Reduction In Arrythmias In A New Study With Istaroxime And A Pure SERCA2a Activator
The new preclinical data further supports istaroxime clinical data from three Phase 2 studies Arrythmias can be a serious complication of current rescue therapies for treatment of patients with acute heart failure WARRINGTON, Pa., Jan. 02, 2024 (GLOBE NEWSWIRE) — Windtree Therapeutics, Inc. (“Windtree” or the “Company”) (NasdaqCM: WINT), a biotechnology […]
Pulse Biosciences Announces First-in-Human Procedures with its Novel CellFX™ Nanosecond Pulsed Field Ablation (nsPFA™) Cardiac Catheter
HAYWARD, Calif., December 20, 2023–(BUSINESS WIRE)–Pulse Biosciences, Inc. (Nasdaq: PLSE), a company primarily focused on leveraging its novel and proprietary CellFX Nanosecond Pulsed Field Ablation (nsPFA) technology for the treatment of atrial fibrillation, today announced the completion of the first five procedures in its first-in-human feasibility study with its novel […]
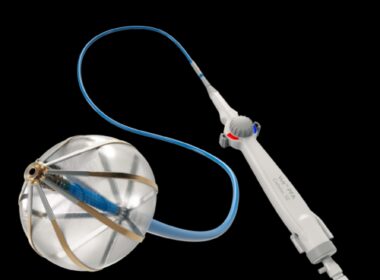
Medtronic creates history with FDA approval of its novel PulseSelect™ Pulsed Field Ablation System to treat atrial fibrillation
Safe, efficient, and effective treatment for both paroxysmal and persistent atrial fibrillationDUBLIN, Dec. 13, 2023 /PRNewswire/ — Medtronic plc (NYSE: MDT), a global leader in healthcare technology, today announced that the United States Food and Drug Administration (FDA) has approved the PulseSelect Pulsed Field Ablation (PFA) System for the treatment of both paroxysmal and persistent atrial fibrillation (AF). This is the first PFA technology to receive FDA approval and follows the recent European CE (Conformité Européenne) Mark of the PulseSelect PFA system in November.
Continue Reading
PulseSelect
PulseSelect PFA System
“Launching the first FDA-approved PFA technology is not just a milestone; the PulseSelect PFA system is setting a new standard in safety for AF ablation with excellent efficacy and efficiency1. It’s a major step towards fulfilling our vision of providing disruptive electrophysiology solutions for patients,” said Rebecca Seidel, SVP and President of the Cardiac Ablation Solutions business, which is part of the Cardiovascular Portfolio. “The PulseSelect PFA system, together with the CE Marked Affera™ mapping and ablation system and our strong Cryo platform, enables us to provide a broad portfolio of solutions to clinicians and their patients, all developed with years of research and supported by compelling scientific evidence.”
The PulseSelect PFA system was engineered with differentiated safety features and provides rapid, effective pulmonary vein isolation (PVI) through consistent and predictable energy delivery and catheter maneuverability. The system is designed to enable a seamless transition to PFA in a clinician’s preferred workflow2. The PulseSelect PFA system’s safety, efficacy, and efficiency is supported by the PULSED AF study, which showed a 0.7% safety event rate and clinical success rates of 80% in both paroxysmal and persistent AF patients1.
“The PulseSelect PFA system ushers the EP community to a new era of safe, effective, and efficient AF ablation that overcomes many challenges in our current practice,” said Dr. Amin Al-Ahmad, clinical cardiac electrophysiologist at St. David’s Medical Center in Austin, TX and one of 67 global operators in the PULSED AF trial. “In my clinical experience with the catheter, it was designed for AF ablation procedures. The learning curve in using the catheter and system is short, and the catheter enables the operator to deliver fast and controlled pulsed field energy for AF ablation.”The PulseSelect PFA system also includes the following:
Designed as a plug-and-play system, PulseSelect can be used with any mapping system or with just fluoroscopy2.
Built-in safety features such as a phrenic nerve test pulse, a non-therapeutic low voltage pulse that provides a preemptive assessment of catheter proximity to the phrenic nerve prior to delivering a therapeutic application.
Fixed spacing for the nine-electrode catheter, which produces a predictable and consistent electric field for contiguous ablation2. In addition to ablation, the nine electrodes can also be used for pacing and sensing.
The small, 9Fr bidirectional catheter enhances maneuverability and access to various anatomical structures and is compatible with a 10Fr sheath, including the custom bidirectional FlexCath Contour™ sheath.
“We are thrilled to see the continuous innovation of our legacy Cryoablation portfolio alongside the approval of the PulseSelect PFA system in the U.S.,” said Khaldoun Tarakji, MD MPH, Chief Medical Officer of the Cardiac Ablation Solutions business at Medtronic. “Every patient deserves the best care. What motivates all of us at Medtronic is the privilege of serving patients by empowering electrophysiologists globally with the safest and most effective ablation technologies that seamlessly integrate with their workflows and enable them to tailor therapy based on their patients’ needs.”The PulseSelect PFA system is also the first FDA Breakthrough-designated PFA technology to be approved. The designation is intended to help patients gain more timely access to medical devices that have the potential to make a significant impact in the diagnosis or treatment of life-threatening conditions.Commercialization of the PulseSelect PFA system will start in early 2024.About Atrial Fibrillation and Pulsed Field AblationAF is one of the most common and undertreated heart rhythm disorders, affecting more than 60 million people worldwide3. AF is a progressive disease, meaning it can become worse over time and can increase the risk of serious complications including heart failure, stroke and increased risk of death.4-7 Current ablation technologies rely on thermal effects to target cardiac tissue and risk damage to additional collateral structures in the heart. PFA is a breakthrough ablation technology that uses pulsed electric fields to efficiently isolate the pulmonary veins for the treatment of AF. Because the mechanism of cell death is non-thermal, the risk of collateral structure damage is potentially lower. About MedtronicBold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin, Ireland, is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 95,000+ passionate people across 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE:MDT), visit www.Medtronic.com, and follow @Medtronic on LinkedIn.Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic’s periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
Verma A, et al. Circulation. 2023;147:1422-1432
Medtronic data on file. November 2023.
Roth GA, Mensah GA, Johnson CO et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol 2020;76:2982-3021.
Miyasaka Y, Barnes ME, Bailey KR, et al. Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. J Am Coll Cardiol 2007;49:986-92.
Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2020.
Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983-8.
Lubitz SA, Moser C, Sullivan L, et al. Atrial fibrillation patterns and risks of subsequent stroke, heart failure, or death in the community. J Am Heart Assoc 2013;2:e000126
Contacts:
Allison Kyriagis
Ryan Weispfenning
Public Relations
Investor Relations
+612-750-6061
+1-763-505-4626
SOURCE Medtronic plc
Lucem Health Introduces Reveal for Stroke to Accelerate Treatment of AFib-Related Stroke Risks
Groundbreaking Solution Identifies Undiagnosed AFib and Speeds Treatments Proven to Prevent Strokes
DAVIDSON, N.C., Dec. 13, 2023 /PRNewswire/ — Lucem Health™, a leader in AI-driven early disease detection solutions, announced today the release of Reveal for Stroke, an innovative solution designed to identify patients with undocumented atrial fibrillation (AFib) and higher risk of AFib-related strokes. As noted by the American Heart Association, AFib affects 2.7-6.1 million individuals in the US and significantly increases the risk of ischemic stroke. Thus, the need for early identification and intervention has never been more critical.
AFib-related strokes constitute approximately 15-20% of all ischemic strokes. However, at least one-in-five AFib cases remain undocumented in clinical practice, leading to many patients missing out on important stroke prevention treatment. Reveal for Stroke aims to close this gap by accurately identifying patients with previously undiagnosed AFib and Atrial Flutter, thereby enabling timely preventive care.
Reveal for Stroke leverages existing ECG and other clinical data that already exists in patient records. The solution identifies adults ages 22 and above with undiagnosed AFib or Atrial Flutter and calculates each patient’s CHA2DS2-VASc score to assess stroke risk comprehensively. Reveal for Stroke enables timely and appropriate treatment by presenting clinicians with an FDA-cleared AccurECG™ analysis, stroke risk scores, and HAS-BLED assessments for major bleeding risks – all vital data points for making informed, effective clinical decisions.
Remarkably, Reveal for Stroke surfaces up to 95% of previously unidentified AFib patients. By getting patients on the right treatment pathways–which may include blood thinners, ablation therapy, and implantables–stroke risk can be reduced by about 70% in patients with AFib.
“At Lucem Health, we develop solutions that identify patients with major health risks that are hiding in plain sight. Reveal for Stroke reflects that commitment by bringing actionable stroke risk insights into everyday practice, highlighting patients who need care and thus saving lives, without changing clinical workflows,” says Jeremy E. Pierotti, General Manager of Solutions at Lucem Health.
Lucem Health has partnered with AccurKardia, a renowned ECG-led diagnostics company, to develop Reveal for Stroke. AccurKardia’s proprietary AccurECG™ Analysis System is an FDA-cleared, HIPAA-compliant, device-agnostic, and fully automated ECG interpretation software solution. This state-of-the-art system is capable of detecting 13 different arrhythmias with exceptional accuracy, empowering healthcare organizations to effectively prioritize patients for early interventions.
“Reveal for Stroke represents a shared vision with Lucem Health to harness the full potential of ECG data in managing stroke risks. Our combined efforts have produced an easy-to-implement tool that is as clinically impactful as it is user-friendly, marking an advance in patient-centric care,” says Juan C. Jimenez, CEO of AccurKardia.
About Lucem Health Lucem Health helps healthcare providers accelerate disease detection and treatment using practical, responsible AI, so they can improve patients’ lives and increase the clinical and financial yield from today’s scarce care delivery resources. We envision a world in which clinicians detect problems before they become life-threatening and patients get world-class care, everywhere. Learn more at www.lucemhealth.com.
About AccurKardia AccurKardia is an ECG-led diagnostics software company unlocking the value of the ECG signal for broad diagnostics coverage and disease management ultimately improving clinical outcomes and saving lives at a planetary scale. AccurKardia’s first FDA-cleared product, AccurECG (Class II SaMD), is a cloud-based and device-agnostic software for fully automated ECG interpretation and detection for up to 13 arrhythmias. The technology assists cardiac monitoring companies in the analysis of ECGs recorded from holters, event recorders, and cardiac telemetry devices. The Company completed Cohort 3 of Mayo Clinic Platform_Accelerate in July 2023. For more information about AccurKardia, please visit: https://www.accurkardia.com/.
Media Contacts:Lucem HealthOscar Eclov-ReherDirector of Marketing[email protected]
AccurKardiaNicole Brunetnicole@wearesubstrate.com
SOURCE Lucem Health
Acesion Pharma announces Nature Medicine publication of clinical results in Atrial Fibrillation
AP30663 achieved proof of mechanism with first-in-class SK channel inhibition compound in atrial fibrillation – primary endpoint met: 99.9% probability of superiority over placebo
AP31969, a novel compound being developed for sinus rhythm maintenance in atrial fibrillation, commences dosing in phase 1
COPENHAGEN, Denmark, Dec. 13, 2023 /PRNewswire/ — Acesion Pharma (“Acesion” or “the Company”), a biotech company pioneering first-in-class novel therapies for atrial fibrillation (“AF”), the most common cardiac arrhythmia, today announces the publication of data in Nature Medicine with the full results from its Phase 2 proof-of-concept trial of AP30663, a first-in-class SK ion channel inhibitor for conversion of AF to normal sinus rhythm. As previously announced, the trial met its primary endpoint, thereby demonstrating the first ever proof mechanism for SK channel inhibition as a novel treatment for AF.
AP30663 Phase 2 trial results
In the trial, patients with a current episode of AF lasting for seven days or less were randomized to receive an intravenous infusion of 3 or 5 mg/kg of AP30663 or placebo. The primary endpoint of the trial was cardioversion from AF to sinus rhythm within 90 min from the start of the intravenous infusion, analysed using Bayesian statistics.
The primary endpoint occurred in 42% (5 of 12), 55% (12 of 22) and 0% (0 of 25) of patients treated with 3 mg/kg AP30663, 5 mg kg/1 AP30663, or placebo, respectively. Both doses demonstrated more than 99.9% probability of superiority over placebo, surpassing the prespecified 95% threshold. No ventricular arrhythmias were observed, and adverse event rates were comparable between active and placebo group. The results are now published in the high-impact journal Nature Medicine (https://www.nature.com/articles/s41591-023-02679-9).
AP31969 Phase 1 trial initiated
In addition to the publication of the above phase 2 trial results, the company has also successfully dosed the first healthy volunteer subjects in a phase 1, first-human-dose, clinical trial of AP31969, an oral first-in-class SK ion channel inhibitor designed to maintain sinus rhythm in patients suffering from AF.
The phase 1 trial is planned to consist of two parts: single ascending dose and multiple ascending dose. In addition, the effect of food on the absorption of the compound will be investigated. Results from the trial are expected in Q3 2024.
AP31969 is being developed for chronic oral maintenance treatment to prevent AF recurrence (maintain sinus rhythm) and has shown strong pre-clinical efficacy combined with a promising safety profile.
Anders Gaarsdal Holst, MD, PhD, Chief Executive Officer of Acesion, said “We are pleased that Nature Medicine has recognised the importance of our AP30663 results for conversion of AF to sinus rhythm and accepted them for publication in their prestigious journal. Based on this achievement of clinical proof-of-mechanism, and enabled by our recent successful Series B financing, we have now also moved our oral SK inhibitor AP31969 into clinical development. Through this study, we will learn about the safety, as well as the pharmacokinetics of AP31969 and it is an important step towards addressing the large unmet need within atrial fibrillation.”
AF is the most common type of cardiac arrhythmia and is forecast to affect 25 million people in the US and EU by 2030. Existing drug therapies for AF are associated with the risk of serious cardiac or other adverse effects, resulting in a great need for safer drugs. Yet, there has been a lack of innovation and development with no new chronic AF drug approved for nearly 20 years. With AP31969, Acesion is aiming to develop a safer alternative.
About Acesion Pharma
Acesion builds on 20 years of know-how with development of small-molecule SK inhibitors and is the world leader in the field of SK channel inhibition, being the only company able to identify and progress SK channel inhibitors into clinical trials. In pre-clinical studies, inhibiting the SK channels has been shown to result in pronounced antiarrhythmic effects in the atria while avoiding effects on the ventricles, the major chambers of the heart and the source of most safety issues with existing drugs. Furthermore, the SK channel has strong genetic validation, with genes encoding the SK channels having one of the strongest associations to AF in human genome-wide association studies. Acesion’s AP30663 IV is a short acting conversion therapy that has completed a phase 2 trial proving the value of this first-in-class mechanism in AF and thereby de-risking Acesion’s broader SK inhibitor pipeline. Acesion’s oral program with lead candidate AP31969 is designed and engineered using in house knowhow to optimise for, and meet, very high hurdles in both efficacy and particularly safety where existing treatments fall short of patient needs. Acesion Pharma is backed by Novo Holdings, Canaan, Alpha Wave Ventures, Global BioAccess Fund, Wellcome Trust, Broadview Ventures and FC Capital.
https://www.acesionpharma.com/
About atrial fibrillation (AF)
AF is the most common type of cardiac arrhythmia mainly affecting the elderly population. Lifetime risk for development of AF is estimated at more than one in three. It is forecast to affect 25 million people in the US and EU by 2030. AF is characterized by chaotic electrical activity in the upper chambers of the heart, the atria, resulting in an irregular and high heart rate. AF is associated with impaired quality of life, increased rate of hospitalization, and a five-fold increased risk of stroke. Increasing evidence suggests that patients with AF also face a higher risk of cognitive dysfunction and dementia.
AF is often treated by electrical shock to bring the heart back to its normal rhythm (conversion). This requires general anesthesia in a hospital setting. In addition, many patients are likely to benefit from chronic treatment to prevent AF and maintain normal sinus rhythm. Existing drug therapies for cardioversion or prevention of AF are associated with risk of serious cardiac or other adverse effects, resulting in a great need for safer drugs. Yet, there has been a lack of innovation and development with no new chronic AF drug approved for nearly 20 years. A landmark New England Journal of Medicine published clinical trial (https://doi.org/10.1056/NEJMoa2019422) has shown that sinus rhythm maintenance treatment improves survival and long-term outcomes for AF patients.
SOURCE Acesion Pharma