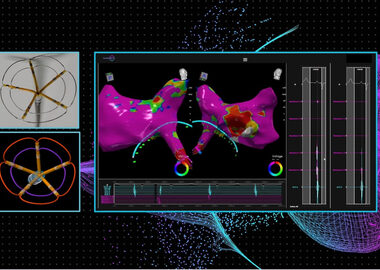
ST. LOUIS, Oct. 31, 2023 (GLOBE NEWSWIRE) — Stereotaxis (NYSE: STXS), a pioneer and global leader in surgical robotics for minimally invasive endovascular intervention, today announced the first patients in the United States have been treated successfully utilizing Abbott’s EnSite™ X EP System integrated with Stereotaxis’ Robotic Magnetic Navigation System. […]
Rhythm
Pulsecare Medical’s nsPFA clinical trial receives satisfactory short-term follow-up results
SHENZHEN, China, Oct. 30, 2023 /PRNewswire/ — Pulsecare Medical, an innovative minimally invasive and non-invasive therapeutic technology company, announced today that its nanosecond pulsed field ablation (nsPFA) system for cardiac electrophysiology, the world’s first third-generation…
SentiAR Announces Second FDA Clearance for CommandEP™ Interface
ST. LOUIS, Oct. 12, 2023 /PRNewswire/ — SentiAR, Inc. a St. Louis, Missouri based pioneer in using Augmented Reality (AR) visualization technology for medical procedures has received their second FDA 510K clearance CommandEP™ adding a new integration. CommandEP™ integrates existing 3D cardiac mapping systems to create a real time 3D holographic interface that provides physicians with an […]
Rejuvenate Bio Announces Preclinical Data for Gene Therapy Candidate RJB-0402 for the Treatment of Arrhythmogenic Cardiomyopathy
SAN DIEGO–(BUSINESS WIRE)–Rejuvenate Bio, today announced new preclinical efficacy data for RJB-0402, a gene therapy targeting key disease-mediating pathways. RJB-0402 demonstrated efficacy in a mouse model of arrhythmogenic cardiomyopathy (ACM), an inherited disease caused by mutations in one of several genes encoding desmosomal proteins. A single dose of RJB-0402 was […]
Biosense Webster Study Links Lower Risk of Heart Failure in Atrial Fibrillation Patients Treated with Catheter Ablation Compared to Antiarrhythmic Drugs
Irvine, CA October 5, 2023 – Biosense Webster, Inc. the global leader in cardiac arrhythmia treatment and part of Johnson & Johnson MedTech,1 announced today new data from a Biosense Webster funded study which sought to compare the risk of heart failure incidence among atrial fibrillation (AFib) patients treated with […]
Pulse Biosciences Announces Collaboration with CardioNXT for nsPFA First-in-Human Atrial Fibrillation Study
HAYWARD, Calif.–(BUSINESS WIRE)–Pulse Biosciences, Inc. (Nasdaq: PLSE, “the Company”), a company primarily focused on leveraging its novel and proprietary Nanosecond Pulsed Field Ablation (nsPFA) technology for the treatment of atrial fibrillation, today announced a collaboration with CardioNXT, to support Pulse Biosciences’ planned nsPFA cardiac catheter first-in-human study focused on the […]
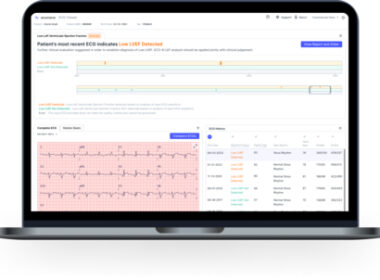
Anumana Receives U.S. FDA 510(k) Clearance for ECG-AI Algorithm to Detect Low Ejection Fraction
CAMBRIDGE, Mass.–(BUSINESS WIRE)–Anumana, Inc., a leading AI-driven health technology and nference portfolio company working in collaboration with Mayo Clinic, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance for ECG-AI LEF, a breakthrough artificial intelligence (AI)-powered medical device to detect low ejection fraction (LEF) in patients at risk of heart failure. “Anumana’s […]
iRhythm Launches Next Generation Zio Monitor and Enhanced Zio Service: Its Smallest, Lightest and Thinnest Cardiac Monitor
SAN FRANCISCO, Sept. 26, 2023 (GLOBE NEWSWIRE) — iRhythm Technologies, Inc. (NASDAQ:IRTC) today announced the U.S. launch of its next-generation Zio® monitor and enhanced Zio® long-term continuous monitoring (LTCM) service. Zio monitor is iRhythm’s smallest, lightest and thinnest cardiac monitor, enhancing the cardiac monitoring experience for patients and healthcare providers together with new service […]
Kestra Medical Technologies Offers Electrophysiology and Cardiology Clinicians Opportunity to Wear ASSURE WCD System at #HRX2023 Conference
SEATTLE–(BUSINESS WIRE)–Kestra Medical Technologies is launching the ASSURE Clinician Experience (ACE), a program that lets electrophysiology and cardiology professionals wear and simulate use of the ASSURE® Wearable Cardioverter Defibrillator (WCD) system at The Heart Rhythm Society #HRX2023 global health and technology conference, September 21-23, 2023 in Seattle, WA. Clinicians that participate will be […]
Orchestra BioMed Granted FDA Approval of IDE to Initiate BACKBEAT Pivotal Study of BackBeat CNT™ for the Treatment of Hypertension in Pacemaker Patients
NEW HOPE, Pa., Sept. 19, 2023 (GLOBE NEWSWIRE) — Orchestra BioMed Holdings, Inc. (Nasdaq: OBIO) (“Orchestra BioMed” or the “Company”), a biomedical company accelerating high-impact technologies to patients through risk-reward sharing partnerships, today announced the U.S. Food and Drug Administration (“FDA”) granted approval of an investigational device exemption (“IDE”) to […]