LAGUNA HILLS, Calif., Sept. 14, 2023 /PRNewswire/ — Adagio Medical, Inc., a leading innovator in catheter ablation technologies for atrial fibrillation (AF) and ventricular tachycardia (VT), announced the first ultra-low temperature cryoablation (ULTC) procedure performed using the Adagio vCLAS™ catheter system in the United States as part of the FULCRUM-VT (Feasibility of Ultra-Low Temperature Cryoablation for Recurring Monomorphic Ventricular Tachycardia) clinical trial, NCT # […]
Rhythm
Implicity CEO Selected to Participate in HRX 2023 Panel Discussion on AI & Remote Cardiac Monitoring
CAMBRIDGE, Mass, Sept. 18, 2023 /PRNewswire/ — Implicity, a leader in remote patient monitoring (RPM) and cardiac data management solutions, announced today that co-founder and CEO Arnaud Rosier, MD, PhD, has been selected to participate in a panel discussion at the upcoming HRX 2023 Digital Health Event in Seattle, WA. The discussion, AI-aided Remote Interventions – Will […]
Field Medical, Inc. Announces Oversubscribed Seed Round to Advance Next-Generation Cardiac Ablation Technology
CARDIFF-BY-THE-SEA, Calif., Sept. 18, 2023 /PRNewswire/ — Field Medical, Inc. (Field Medical), an emerging leader in cardiac catheter ablation, announced today that it has closed its oversubscribed seed round with investments totaling $14M. The convertible note funding was led by private investors who were joined by multiple strategic investors. This financing will support preclinical-to-clinical […]
Atrial Fibrillation Study with Abelacimab Stopped Early by the Data Monitoring Committee Due to an Overwhelming Reduction in Bleeding as Compared to a DOAC (Direct Oral Anticoagulant)
CAMBRIDGE, Mass., September 18, 2023 (BUSINESS WIRE)– Anthos Therapeutics, Inc., a clinical stage company developing innovative therapies for cardiovascular diseases, founded by Blackstone Life Sciences, announced today that the AZALEA-TIMI 71 Phase 2 study in 1,287 patients with atrial fibrillation at moderate-to-high risk of stroke, met its primary endpoint. The […]
SmartCardia Receives FDA Clearance for its 14-Day 7-Lead ECG Real-Time Patch and Cloud Platform
LAUSANNE, Switzerland, Sept. 15, 2023 /PRNewswire/ — SmartCardia has received FDA clearance for its 7-lead real-time ECG monitoring patch and cloud platform. SmartCardia’s 7L patch is easy-to-wear, cable-free, waterproof and can be used for continuous monitoring for up to 14 days. SmartCardia 7L Patch SmartCardia Cloud Dashboard “SmartCardia’s 7L patch and cloud platform […]
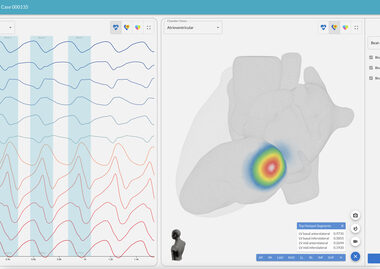
Vektor Medical Unveils Enhanced vMap Technology for Accurate, Non-invasive Mapping of Cardiac Arrhythmias
SAN DIEGO–(BUSINESS WIRE)–Vektor Medical, developer of the only technology to accurately map arrhythmias using just 12-lead ECG data, today announced the release of a series of software enhancements to its AI-based non-invasive solution, vMap. Designed to improve ablation outcomes and procedural efficiencies, the newly updated vMap software integrates additional automation […]
Boston Scientific Announces FDA Approval for the Latest-Generation WATCHMAN FLX™ Pro Left Atrial Appendage Closure Device
MARLBOROUGH, Mass., Sept. 6, 2023 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) today announced it has received U.S. Food and Drug Administration approval for the latest-generation WATCHMAN FLX™ Pro Left Atrial Appendage Closure (LAAC) Device. Designed to further advance the procedural performance and safety of the WATCHMAN technology, which is indicated to reduce stroke […]
HeartSciences Signs Distribution Agreement with FJ Medical, Denmark
Southlake, TX, Aug. 31, 2023 (GLOBE NEWSWIRE) — Heart Test Laboratories, Inc. d/b/a HeartSciences (Nasdaq: HSCS; HSCSW) (“HeartSciences” or the “Company”), an artificial intelligence (AI)-based medical technology company focused on transforming ECGs/EKGs to save lives through earlier detection of heart disease, today announced that it has signed a distribution agreement with FJ […]
RhythmScience Announces Formation of Medical Advisory Board
SAN FRANCISCO, Aug. 31, 2023 /PRNewswire/ — RhythmScience, a leader in cardiac data management and services, today announced the formation of its Medical Advisory Board (MAB). Comprised of renowned experts across heart failure and rhythm management, the MAB will provide invaluable clinical guidance and strategic insights to further advance RhythmScience’s mission of […]
Aziyo Biologics Announces Publication of a CanGaroo® Case Study Demonstrating Bioenvelope Benefits for Reoperative Procedures
SILVER SPRING, Md., Aug. 30, 2023 (GLOBE NEWSWIRE) — Aziyo Biologics, Inc. (Nasdaq: AZYO) (“Aziyo”), a company that develops and commercializes biologic products to improve compatibility between medical devices and the patients who need them, today announced the publication of a case report highlighting results that demonstrate the potential benefits […]