Expert judges determining the best of the best in healthcare innovation recognized OMRON Healthcare for its blood pressure monitors with AI-powered AFib detection HOFFMAN ESTATES, Ill., Oct. 28, 2025 /PRNewswire/ — Judges in the prestigious Digital Health Hub Foundation 2025 Digital…
Rhythm
Stereotaxis Announces EU Launch and 510(k) Submission for Synchrony System to Modernize Interventional Cath Labs
ST. LOUIS, Oct. 15, 2025 (GLOBE NEWSWIRE) — Stereotaxis (NYSE: STXS), a pioneer and global leader in surgical robotics for minimally invasive endovascular intervention, today announced it obtained CE Mark in Europe and submitted a 510(k) application to the FDA in the US for the Synchrony™ system. Synchrony is designed to digitize and modernize the interventional cath lab. Synchrony’s slim and stunning 55” 4K ultra-high-definition display consolidates the viewing and control of all disparate systems in the lab, offering an enhanced procedure experience with custom layouts, streamlined workflows, an intuitive user interface, and a decluttered environment. Synchrony digitizes the video streams with full fidelity and less than a single frame latency, offering crystal-clear visualization. Its architecture allows obsolescence protection for labs as new technologies are introduced in the future. Synchrony is made available with SynX™, a cloud-based HIPAA and GDPR-compliant app that allows for secure remote connectivity, collaboration, recording, and monitoring of the cath lab. “In my role managing the technology across our electrophysiology labs and advising labs across the country, I have significant experience with the various interventional lab display offerings,” said Matthew Dare, CEPS, Research and Technology Coordinator, Texas Cardiac Arrhythmia Institute at St. David’s Medical Center in Austin, Texas. “The underlying hardware and software architecture of Synchrony and SynX is far beyond what anyone else has developed. It promises a better intraoperative experience for physicians and nurses, improved equipment reliability and maintenance, and attractive tools for managing a cardiovascular program with remote monitoring, collaboration and recordings.” “We have long recognized the importance of remote connectivity and collaboration for our EP labs,” said Dr. Jim Cheung, Professor of Medicine and Director of Cardiac Electrophysiology Fellowship and Research at Weill Cornell Medicine in New York City. “Technologies to date though have not made reliable high-quality connectivity easy. SynX provides a much-needed solution that can permit seamless collaboration across labs and enhance our ability to ensure the best patient care and to train the next generation of physicians.” Synchrony and SynX have been engineered to be foundational platforms for future innovations. The advanced architecture enables future applications and the leveraging of artificial intelligence for enhanced clinical insights, automation, and safety. “Synchrony and SynX are central to our digital surgery efforts to modernize the interventional lab with enhanced workflow, remote connectivity, and smart AI capabilities,” said David Fischel, Stereotaxis Chairman and CEO. “The technology improves the robotic cockpit, and will be critical in supporting future robotic efforts for remote long-distance procedures and automated catheter navigation. The opportunity is much broader than robotic labs as we believe all cath labs stand to benefit from improved workflow, connectivity, and collaboration.” About StereotaxisStereotaxis (NYSE: STXS) is a pioneer and global leader in innovative surgical robotics for minimally invasive endovascular intervention. Its mission is the discovery, development and delivery of robotic systems, instruments, and information solutions for the interventional laboratory. These innovations help physicians provide unsurpassed patient care with robotic precision and safety, expand access to minimally invasive therapy, and enhance the productivity, connectivity, and intelligence in the operating room. Stereotaxis technology has been used to treat over 150,000 patients across the United States, Europe, Asia, and elsewhere. For more information, please visit www.stereotaxis.com. This press release includes statements that may constitute “forward-looking” statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Act of 1934, including statements regarding the completion of the Company’s offering and the anticipated use of proceeds therefrom, usually containing the words “believe”, “estimate”, “project”, “expect” or similar expressions. Forward-looking statements inherently involve risks and uncertainties that could cause actual results to differ materially. Factors that would cause or contribute to such differences include, but are not limited to, the Company’s ability to manage expenses at sustainable levels, acceptance of the Company’s products in the marketplace, the effect of global economic conditions on the ability and willingness of customers to purchase its technology, competitive factors, changes resulting from healthcare policy, dependence upon third-party vendors, timing of regulatory approvals, the impact of pandemics or other disasters, and other risks discussed in the Company’s periodic and other filings with the SEC. By making these forward-looking statements, the Company undertakes no obligation to update these statements for revisions or changes after the date of this press release. There can be no assurance that the Company will recognize revenue related to customer purchase orders and other commitments because some of these purchase orders and other commitments are subject to contingencies that are outside of the Company’s control and may be revised, modified, delayed, or canceled. Stereotaxis Contacts: David L. FischelChairman and Chief Executive Officer Kimberly PeeryChief Financial Officer 314-678-6100Investors@Stereotaxis.com
Adagio Medical Unveils Preliminary Acute Results from FULCRUM-VT U.S. Pivotal Study in Late Breaking Session at VT Symposium
97% Acute Effectiveness and Favorable Safety Results from Proprietary ULTC for Ventricular Tachycardia LAGUNA HILLS, Calif.–(BUSINESS WIRE)–Adagio Medical Holdings, Inc. (Nasdaq: ADGM) (“Adagio” or “the Company”), a leading innovator in catheter ablation technologies for the treatment of cardiac arrhythmias, today announced preliminary acute (within 7 days) safety and efficacy results […]
Pulse Biosciences Announces Presentation of Late-Breaking Data From the nPulse™ Cardiac Surgical System First-in-Human Feasibility Study at the 39th European Association for Cardio-Thoracic Surgery Annual Meeting
uccessfully treated atrial fibrillation (AF) in initial 30 patients with the nPulse™ Cardiac Surgical System HAYWARD, Calif.–(BUSINESS WIRE)–Pulse Biosciences, Inc. (Nasdaq: PLSE), a company leveraging its novel nPulse™ technology using its proprietary Nanosecond Pulsed Field Ablation™ (nanosecond PFA or nsPFA™) energy, today announced late-breaking clinical study results from the nPulse™ […]
Viz.ai Launches Viz ACS™ to Accelerate Treatment for Heart Attack and Reduce Unnecessary Activations
SAN FRANCISCO–(BUSINESS WIRE)–Viz.ai, the leader in AI-powered disease detection and intelligent care coordination, today announced the launch of Viz ACS, a new solution in the Viz Cardio™ Suite. Designed to unite the acute coronary syndrome (ACS) care team on a single platform, Viz ACS provides Emergency Department (ED) physicians and […]
Stereotaxis and CardioFocus Collaborate to Advance Robotic Pulsed Field Ablation for Cardiac Arrhythmias
ST. LOUIS and MARLBOROUGH, Mass., Oct. 13, 2025 (GLOBE NEWSWIRE) — Stereotaxis (NYSE: STXS), a pioneer and global leader in surgical robotics for minimally invasive endovascular intervention, and CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for cardiac arrhythmias, today announced they have entered into a Collaboration Agreement to advance robotic Pulsed Field Ablation (PFA) technology towards commercialization.
Pulse Biosciences Announces Clinical Data Highlighting its nPulse™ Cardiac Surgical System to be Presented at the 39th European Association for Cardio-Thoracic Surgery Annual Meeting
HAYWARD, Calif.–(BUSINESS WIRE)–Pulse Biosciences, Inc. (Nasdaq: PLSE), a company leveraging its novel nPulse™ technology using its proprietary Nanosecond Pulsed Field Ablation™ (nanosecond PFA or nsPFA™) energy, today announced the upcoming presentation of early clinical data on the treatment of atrial fibrillation (AF) using the nPulse™ Cardiac Surgical System at the […]
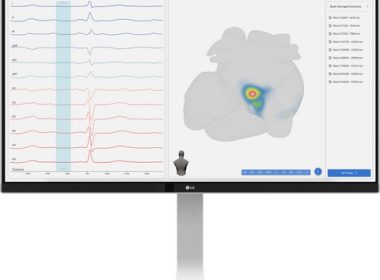
Vektor Medical Secures CE Mark for vMap, Bringing the Benefits of Non-Invasive Arrhythmia Mapping to Europe
SAN DIEGO–(BUSINESS WIRE)–Vektor Medical, a medical technology company transforming cardiac arrhythmia care, announced it has received CE Mark for the vMap® System, a non-invasive tool developed with AI that transforms standard 12-lead ECG data into 3D arrhythmia source maps in under a minute. “vMap is emerging as a simple, non-invasive […]
ACORAI Presents Positive Results in 1,600-Patient CAPTURE-HF Study for its AI-Powered Non-Invasive Multi-Sensor Platform for Improved Heart Failure Patient Management
MINNEAPOLIS, Oct. 6, 2025 /PRNewswire/ — Acorai, a clinical-stage company pioneering non-invasive hemodynamic monitoring for heart failure management, this week announced topline results from the 1,600-patient CAPTURE-HF study (Machine-Learning Estimation of Pulmonary Capillary Wedge and…
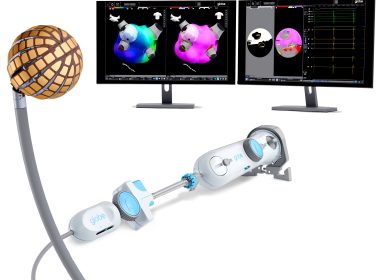
Kardium Announces First Commercial Procedures with the Globe® Pulsed Field System, the World’s Most Advanced Solution for Atrial Fibrillation
VANCOUVER, British Columbia–(BUSINESS WIRE)–Kardium, a private medical device company that’s advancing the way the world treats atrial fibrillation (AF), today announced the successful completion of the first commercial procedures using its integrated mapping and ablation system, the Globe Pulsed Field System, following recent approval from the U.S. Food and Drug Administration (FDA). The […]